Figures & data
Figure 1. CD169+CD8+ T cells are selectively present in the regional LNs and decreased with progressive stages in CRC patients. (A) Immunofluorescence microscopy of tumor-draining LN sections from CRC patients stained with anti-CD169, anti-CD8+ and anti-CD68 monoclonal antibodies. Enlargements show examples of CD169+CD8+ cells (arrowhead) closely associated with CD169+CD68+ cells. (B–E) FACS analysis of CD169+CD8+ T cells in fresh lymphocytes isolated from control donors with hemangioma (n = 2) or congenital megacolon (n = 2) and CRC patients (n = 25). Lymphocytes were gated as CD45+CD3+CD14− cells. Representative dot plots of at least three individuals from more than three independent experiments (B), and the statistical analysis (C–E) of these samples are shown. The continuous and dashed horizontal bars in C and D represent median values. Results are expressed as means ± SEM.
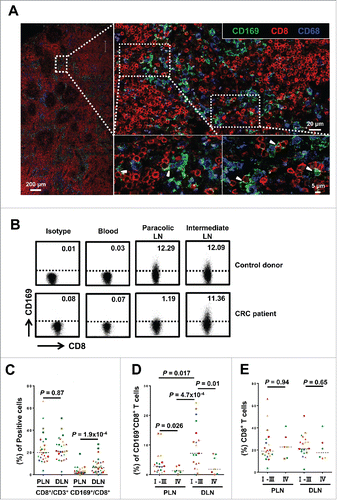
Figure 2. Phenotypic features of CD169+CD8+ T cells in the tumor-draining LN from CRC patients. The phenotypic characteristics of CD169+CD8+ T cells from fresh CRC tissues of peri-LN (PLN, A) or of distant-LN (DLN, B) were determined by flow cytometry. Data for perforin, granzyme B, TNF-α, and IFNγ were acquired in cells stimulated with Leukocyte Activation Cocktail (BD PharMingen, San Diego, CA) at 37°C for 5 h. Results are presented as means ± SEM of three separate experiments. Significant differences are indicated (*p < 0.05; **p < 0.01; ***p < 0.001).
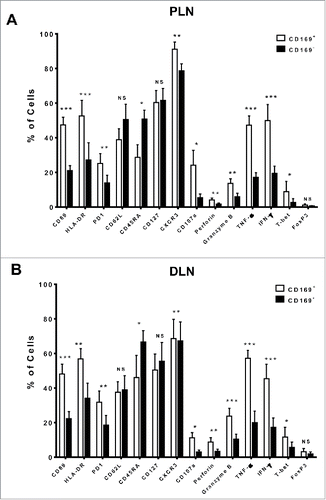
Figure 3. Visualization of target cell killing by co-culture of T cells from tumor-draining LN with tumor cells. (A) FACS analysis of CD169+CD8+ T cells on fresh lymphocytes isolated from peri-LN or distant-LN tissues of CRC patients (upper). Lymphocytes were gated as CD45+CD3+CD14− cells. T cells (effector cell, E) from tumor-draining LN were assessed for non-specific cytotoxicity against the HCT116 colorectal cancer cell line (target cells, T) in vitro by a CFSE-based assay (E:T = 5:1). PI staining was used to discriminate viable and intact cells from dead subpopulations. Cells were co-cultured and harvested for analysis after 5 h of incubation. (B) The histograms depict the viable PI positive target dead cell subpopulations. Results are representative of at least three different CRC patients. Paired t-test (*p < 0.05; **p < 0.01; ***p < 0.001). (C) T cells isolated from distant-LN stained with Cell Tracker™ Red were mixed with T cells from peri-LN and then co-cultured with target cells (HCT116) in vitro. T cell-mediated killing of target cells was monitored by time-lapse epifluorescence and bright field microscopy. The combination of bright field and fluorescence microscopy allowed the detection of interactions between T cells and target cells.
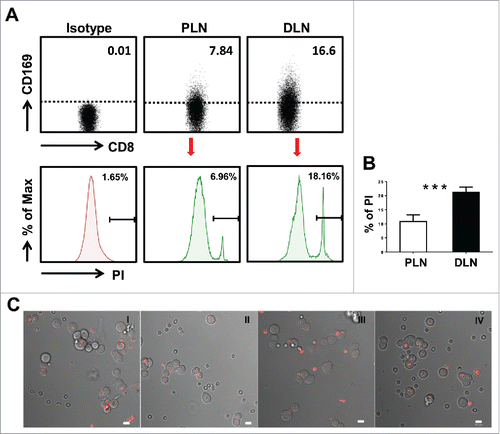
Figure 4. Prognostic significance of CD169+CD8+ T cells in CRC patients. (A) (Left) A representative example of CD169 and CD8+ immunofluorescence staining of a tumor-draining LN section from CRC patients. CD8+ T cells (red), CD169 cells (green), and nuclei (blue) are shown. (Right) Digital image analyzed with the Nuance image software. (B–D) Cumulative OS and DFS times were calculated by the Kaplan–Meier method and analyzed by the log-rank test. The patients were divided into two groups according to the median value of CD169+CD8+ T cells in peri-LN (B), distant-LN (C), and combined regions (D).
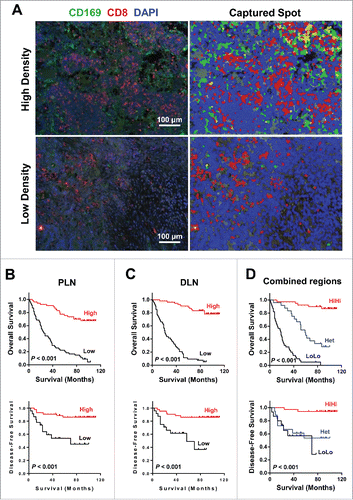
Table 1. Characteristics of patients.
Table 2. Association of CD169+CD8+ T cells density with clinicopathologic characteristics.
Table 3. Univariate and multivariate analyses of factors associated with survival and recurrence.
