Figures & data
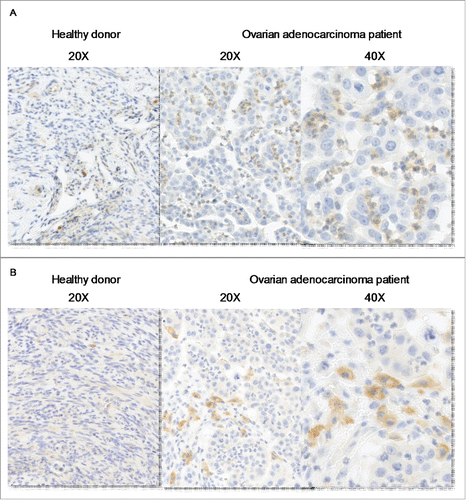
Figure 1. CD39 is more expressed in M-CSF-Mϕ and TAM from ovarian cancer patients compared to GM-CSF-Mϕ (A) The expression of CD39 was analyzed by flow cytometry on GM-CSF-Mϕ, M-CSF-Mϕ and TAM isolated from patients. Results are expressed in relative fluorescent intensity (RFI values are the ratio of antibody/isotype control antibody) (mean ± SEM, n = 11 for M-CSF-Mϕ, GM-CSF-Mϕ and n = 5 for TAM), *p < 0 .05 compared to GM-CSF-Mϕ (B) M-CSF-Mϕ polarization results in the acquisition of CD39 expression whereas GM-CSF-Mϕ polarization results in its maintenance. CD39 expression was analyzed by flow cytometry. Results are expressed in RFI values, as mean ± SEM (n = 4 for monocytes, n = 11 for GM-CSF-Mϕ and n = 11 for M-CSF-Mϕ). (C) HPLC analysis of Etheno-ATP (ATP), -ADP (ADP), -AMP (AMP) and -adenosine (Ado) are expressed in percentage compared to the initial dose of ATP (200 µM = 100%) during 20 min. in GM-CSF-Mϕ and M-CSF-Mϕ (mean of two representative donors). In certain conditions, CD39 inhibitor (POM-1) was used at 10 µM.
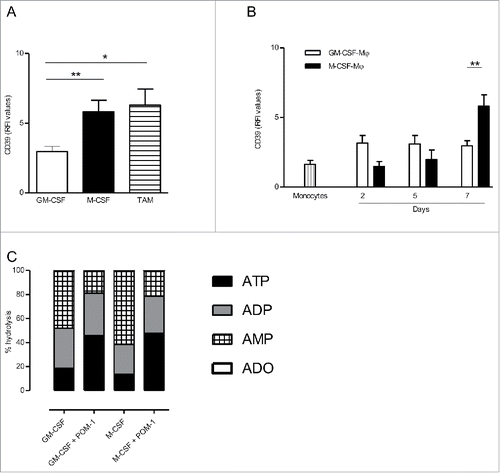
Figure 2. Secretion profile after CD39 blockade with POM-1 in GM-CSF-Mϕ, M-CSF-Mϕ and TAM isolated from patients (A) M-CSF-Mϕ and TAM were stimulated with LPS (200 ng/mL) with or without POM-1 (10 µM), ATP (1 mM) for 6 h. M-CSF-Mϕ and TAM isolated from patients were stimulated with LPS (200 ng/mL) with or without ADA (2.5 UI/mL) for 24 h. (B) GM-CSF-Mϕ were stimulated with LPS (200 ng/mL) with or without POM-1 (10 µM) for 6 h. (A and B) IL-1β secretion in supernatant was analyzed by ELISA (pg/mL). Results are expressed in pg/mL (mean ± SEM, M-CSF-Mϕ: n = 4 for POM-1 and ATP experiments, n = 9 for ADA experiment; TAM: n = 3 for POM-1 and ATP; GM-CSF-Mϕ: n = 5, *p <0.05 compared to LPS alone). (C) M-CSF-Mϕ and TAM isolated from patients were stimulated with LPS (200 ng/mL) with or without POM-1 (10 µM) or ADA (2.5 UI/mL) for 24 h. The secretion of IL-10 was analyzed by ELISA. Results are expressed in ng/mL as mean ± SEM (M-CSF-Mϕ: n = 7 for POM-1 experiment and n = 8 for ADA experiment; TAM: n = 4 for POM-1 experiment and n = 5 for ADA experiment). (D) M-CSF-Mϕ were stimulated with LPS (200 ng/mL) with or without POM-1 (10 µM) during 24 h. Expression of PD-L1 by M-CCSF-Mϕ was analyzed by flow cytometry. Results are expressed in mean ± SEM of percentage of RFI compared to LPS-stimulated M-CSF-Mϕ as 100% (n = 4).
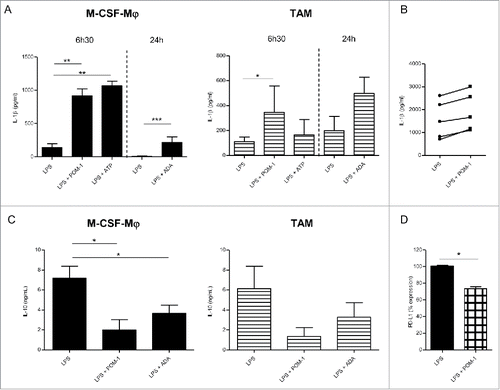
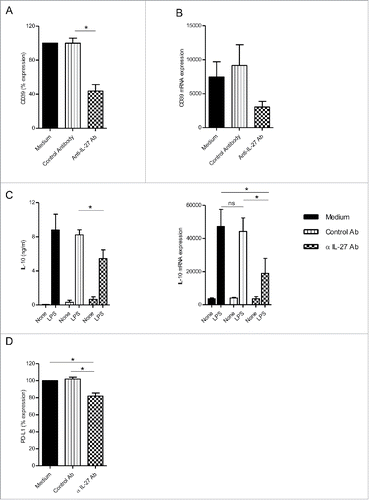
Figure 3. IL-27 blockade during macrophage differentiation in the presence of M-CSF-Mϕ decreased CD39 expression. (A) M-CSF-Mϕ was cultivated during 7 d with M-CSF in the presence of control medium, an anti-IL-27 antibody (5 µg/mL) or its control antibody. CD39 expression was analyzed by flow cytometry. Results are expressed in percent of CD39 expression in monocytes differentiated during 7 d with M-CSF as mean ± SEM (n = 4). (B) CD39 mRNA expression was analyzed by RT-qPCR. Results are expressed in mRNA expression normalized to GAPDH, as mean ± SEM (n = 4). (C, left panel) After stimulation of all type of macrophages generated with LPS (200 ng/mL) during 24 h, the secretion of IL-10 was analyzed by ELISA. Results are expressed in ng/mL as mean ± SEM (n = 5). (C, right panel) IL-10 mRNA expression was analyzed by RT-qPCR. Results are expressed in mRNA expression normalized to GAPDH, as mean ± SEM (n = 4). (D) All type of macrophages generated were stimulated with LPS (200 ng/mL) during 24 h. Expression of PD-L1 by M-CSF-Mϕ was analyzed by flow cytometry. Results are expressed in percentage of RFI compared to LPS-stimulated M-CSF-Mϕ as 100% (n = 5). *p < 0 .05 compared to differentiation process with control antibody or medium alone.