Figures & data
Figure 1. Peripheral NK cells of patients with STS are less cytotoxic than NK cells of RCC patients and HD, but regain cytotoxicity after incubation with IL-2/TKD. NK-specific cytotoxicity against radiolabeled K562 target cells was assessed by 4 h 51Cr release assay. Displayed are the specific lysis values of PBMC/K562 at a ratio of 20:1. (A) NK-specific cytotoxicity of HD (n = 32), STS (n = 24), and RCC patients (n = 4). (B) NK-specific cytotoxicity of STS patients without previous chemotherapy (1st-line, n = 13) compared with STS patients with prior chemotherapy (2nd-line, n = 11). (C) Left panel: NK-specific cytotoxicity of HD (n = 10) and STS patients (1st-line n = 6, 2nd-line n = 6), assessed before and after 96 h of incubation in medium containing interleukin-2 (IL-2) and 14-mer heat shock protein 70 (hsp70) peptide TKD. Only paired samples are shown. Right panel: Normalized data of left panel; relative increase in cytotoxicity for HD, 1st- and 2nd-line STS patients after 96 h of incubation with IL-2 and TKD. For A, B, and C (left panel), box plots represent the median, .75 and .25 percentiles, with whiskers showing minimum and maximum values. Each symbol corresponds to one sample. For C (right panel), mean values with standard errors are shown. For statistical analyses, Kruskal–Wallis tests with Dunn's post hoc tests (A), Mann–Whitney U test (B) and two-way ANOVA with Bonferroni's post hoc test (C) were used.
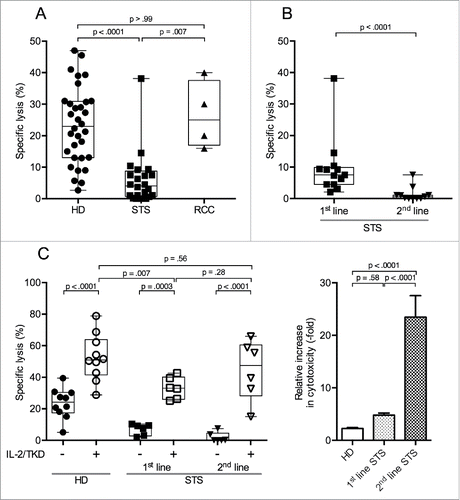
Table 1. Characteristics of STS patients. Median age of 1st-line patients was 44 y, of 2nd-line patients was 34 y. All 2nd-line patients had been treated with anthracyclines before. Months since initial diagnosis indicates the time interval to blood sample withdrawal. TNM stage indicates tumor stage, assessed by CT/MRI imaging, at time of blood withdrawal, with resection status in brackets. Months since last cytostatic treatment indicates the time interval from last application of chemotherapeutic agents to blood sample withdrawal.
Figure 2. Percentage of CD56dim NK cells among PBMCs is reduced and CD16 expression on CD56dim NK cells is diminished in 2nd-line STS patients. (A) Relative distributions of NK cells and NK cell subsets (CD56dim and CD56bright) among PBMCs were assessed by polychromatic flow cytometry. Mean values with standard errors of indicated PBMC subtype cells among live, single, small lymphocytes (FSC/SSC) are shown. Asterisks indicate p values resulting from comparisons of total NK cells (CD3−CD56+ cells) and the CD56dim NK cell subset. Comparison of the percentages of CD56bright NK cells revealed no statistically significant difference. (B, C) show the frequencies of CD16+, CD16low, and CD16− cells among CD56dim and CD56bright NK cells, respectively. Relative distributions are depicted as percentages of the respective NK cell subset, defined as CD3−CD56bright or CD3−CD56dim cells among live, single, small PBMCs (FSC/SSC), as assessed by polychromatic flow cytometry. Box plots represent the median, .75 and .25 percentiles, with whiskers showing minimum and maximum values. Each symbol corresponds to one sample, and comparisons of 1st-line STS (n = 8), 2nd-line STS (n = 5), RCC patients (n = 11) with HD (n = 21) are depicted. For statistical analysis, Kruskal–Wallis test with Dunn's post hoc test was used. *p < .05, ***p < .001.
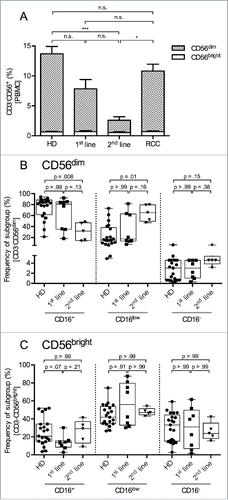
Figure 3. 2nd-line STS patients show reduced percentages of cells expressing the activating NK cell receptor NKG2D and the CD3ζ signaling adaptor protein in peripheral NK cells. (A) Expression of NKG2D (left panel), NKp46 (middle panel), and CD3ζ (right panel) of HD (n = 4), 1st-line STS patients (n = 6), and RCC patients (n = 4) analyzed by polychromatic flow cytometry of uncultured PBMCs. (B) Marker expression in HD (n = 13), 2nd-line STS patients (n = 5) and RCC patients (n = 7). (A–B) Percentages of marker-positive cells among NK cells (CD3−CD56+ cells within live, single, small (FSC/SSC) PBMCs) are depicted. Box plots represent the median, .75 and .25 percentiles, with whiskers showing minimum and maximum values. Each symbol corresponds to one sample. For statistical analyses, Kruskal–Wallis test with Dunn's post hoc tests was used.
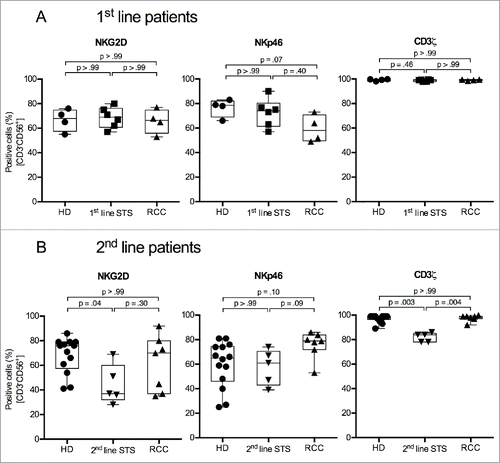
Figure 4. 2nd-line STS patients show reduced percentages of perforin+ and CD57+ cells in peripheral NK cells. (A) Expression of perforin (left panel), granzyme B (middle panel) and CD57 (right panel) of HD (n = 4), 1st-line STS patients (n = 6) and RCC patients (n = 4) analyzed by polychromatic flow cytometry of uncultured PBMCs. (B) Marker expression in HD (n = 13), 2nd-line STS patients (n = 5) and RCC patients (n = 7). (A–B) Percentages of marker-positive cells among NK cells (CD3−CD56+ cells within live, single, small (FSC/SSC) PBMCs) are depicted. Box plots represent the median, .75 and .25 percentiles, with whiskers showing minimum and maximum values. Each symbol corresponds to one sample. For statistical analyses, Kruskal–Wallis test with Dunn's post hoc tests was used.
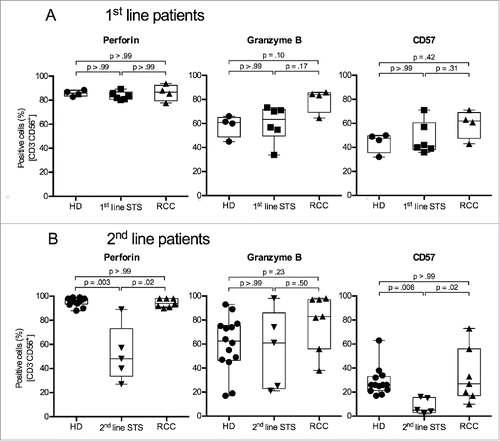
Table 2. Characteristics of RCC patients. Median age of all patients was 65 y. TNM stage indicates tumor stage, assessed by CT/MRI imaging and pathologic diagnosis, at time of blood withdrawal. None of the patients had received systemic treatment or had tumor-related surgery before blood was withdrawn. Patients 1–4 were assessed as comparative group for cytotoxicity assays and flow cytometry assays for 1st-line STS patients, patients 5–11 were assessed as comparative group for flow cytometry assays for 2nd-line STS patients.
