Figures & data
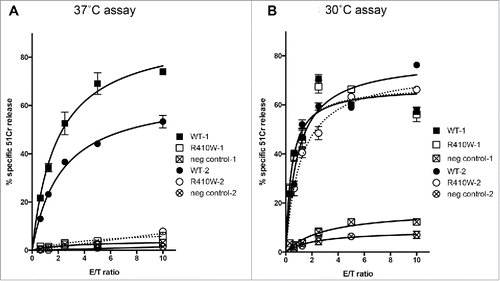
Figure 1. Characterization of PRF1 defects within the family. Family pedigree showing the relationship between family members in which PRF1 analysis was performed. An arrow indicates the index case. Each family member's cancer history is also indicated (H = haematological malignancy; S = solid cancer; DF = disease free; U = unknown cancer history).
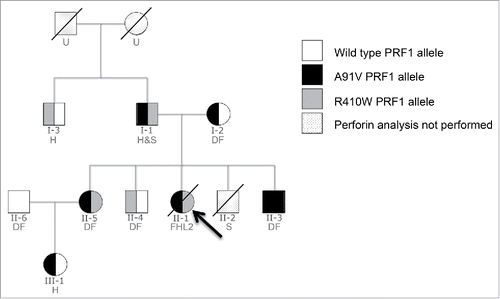
Table 1. Summary of PRF defects and associated disease in family members. Analysis of PRF included PRF1 genotype (assessed by Sanger sequencing) and PRF expression in NK cells (assessed by intracellular FACS). The penetrance of PRF mutations was found to be over 50% with five out of nine carriers affected by disease, including solid tumors.
Figure 2. Intracellular PRF expression in selected family members. Intracellular PRF expression in CD56+CD8− NK cells in patients I-1, II-3, II-5, and II-6 (serving as WT perforin control); in parenthesis is the median fluorescence intensity. Of note, patient II-3, who is homozygous for the A91V mutation but has remained healthy, demonstrates reduced, but appreciable PRF levels.
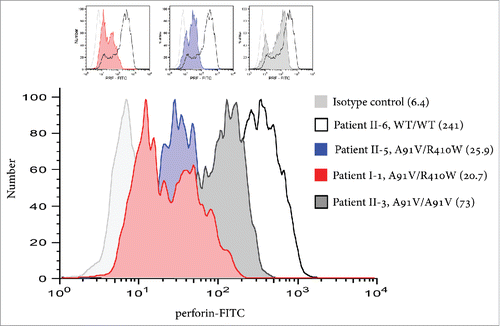
Figure 3. Determining the nature of the R410W mutation. (A) At 37°C, reconstitution of Prf1−/− CTLs with R410W mutant PRF leads to cytolytic activity (assessed by 51Cr release assays) <5% of Prf1−/− CTLs reconstituted with wild-type perforin. (B) At 30°C (a permissive temperature for protein folding),Citation11 Prf1−/− CTLs transfected with R410W mutant PRF have cytolytic activity that is indistinguishable from Prf1−/− CTLs transfected with wild-type PRF. A complete recovery of R410W activity indicates that the only reason for its marginal activity at 37°C is a severe misfolding, and the mutation itself does not affect PRF function.