Figures & data
Figure 1. TRAPs induce IL-10-producing B cell differentiation in vitro and in vivo. (A) Frequencies of IL-10-producing B cells determined by flow cytometry. Splenocytes were incubated with TRAPs (3 μg/mL) or LPS (10 μg/mL) for 72 h. PIM stimulation was performed for 5 h before IL-10 staining. (B) ELISA determinations of IL-10 secretion in culture supernatants were also shown. (C) Phenotypic analysis of IL-10+ (red line) or IL-10− (green line) B cells from TRAP-treated B cell cultures for 72 h by flow cytometry. Gray shaded histograms indicate the isotype staining. (D and E) Mice were i.v. injected with TRAPs (30 μg per mouse) three times with 1 d of intervals and frequencies of splenic IL-10+ B cells were determined 4 d after last treatment (n = 3; as shown in D), CD1d and CD5 expression was assessed by flow cytometry as previously shown. Representative contour plot showing CD1d and CD5 expression on IL-10+ and IL-10− B cells, as shown in E. (F and G), Mice bearing established Atg5 KD 4T1 tumor or control 4T1 tumor were sacrificed (n = 6) when solid tumor volumes reached approximately 150 mm2, and the frequency of IL-10+ B cells in the tumor tissue (as shown in F) and the draining lymph node (as shown in G) was detected. Data (mean ± s.e.m) are representative of three independent experiments. NS: p > 0.05, * p < 0.05, ** p < 0.01, *** p < 0.001 by unpaired t-test (A, B, D, F and G).
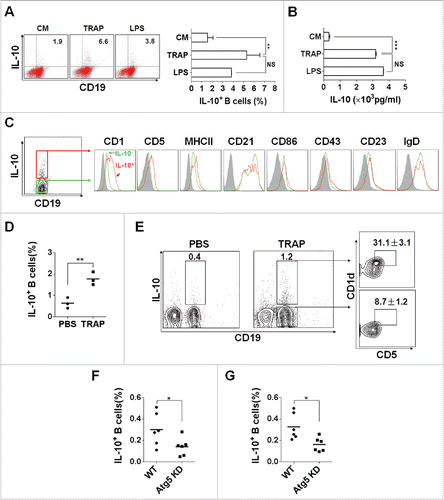
Figure 2. TRAP-induced IL-10-producing B cells suppress T-cell proliferation. (A–D) CFSE-labeled purified CD4+ T cells (A and B) and CD8+ T cells (C and D) were stimulated with plate-bound anti-CD3/anti-CD28 mAb and were either cultured alone (T cells only) or co-cultured with TRAP-induced B cells (3 μg/mL) from WT mice or IL-10−/− mice at ratio of 1:1 for 4 d, un-stimulated T cells were used as control. In the transwell experiment, B cells and T cells were added in the upper and lower chambers, respectively. CFSE dilution was determined by flow cytometry. (E–F) CFSE-labeled splenocytes from naive OT-1 mice were stimulated with OVA257–264 (1 µg/mL) and co-cultured with purified B cells, OVA+ TRAP-induced B cells, or OVA− TRAP-induced B cells at ratio of 1:1. Cells were collected after 4 d, stained with anti-CD8+ mAb and anti-Vβ 5.1/5.2. CFSE dilution was determined by flow cytometry. Results are representative of three independent experiments. NS: p > 0.05, *** p < 0.001 by unpaired t-test (B, D and F).
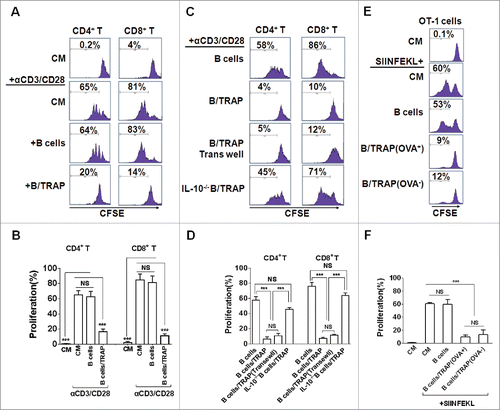
Figure 3. TRAP-induced IL-10-producing B cells inhibit T cell immune response and their antitumor efficacy in a non-antigen specific manner. Tumor-bearing mice (n = 8) were s.c. vaccinated with DCOVA (1 × 106), and then adoptively transferred (i.v.) with E.G7-OVA or B16-F10 cells-derived TRAP-activated B cells (1 × 107). (A-F) At day 7 after last vaccination, the frequencies of the IL-10+ CD19+ B cells in the spleens from mice of each group (n = 3) (A and B) and the IFN-γ+ CD8+ T cells (C and D) and IFN-γ+ CD4+ T cells (E and F) cells in the spleen were determined by flow cytometry. (G) The secretion of IFN-γ by splenocytes when re-stimulated in vitro with OVA was measured by ELISA. (H and I) remaining five mice were continuously monitored and measured tumor volume (H) and percentage of survival (I). Data (mean ± s.e.m) are representative of three independent experiments. NS: p > 0.05, * p < 0.05, ** p < 0.01, *** p < 0.001 by unpaired t-test (B, D, F and G), two-way analysis of variance (H), and log-rank test (I).
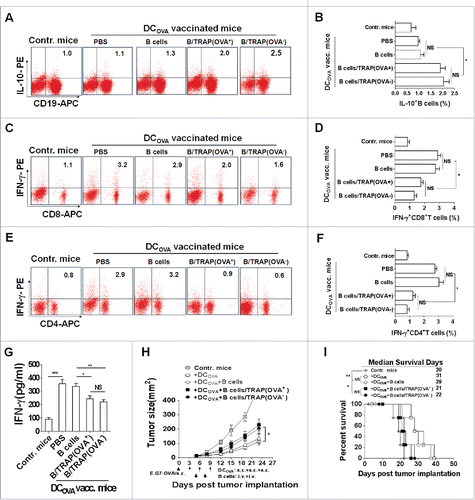
Figure 4. TRAPs induce IL-10-producing B cell via TLR2-MyD88-NF-κB dependent signaling pathway. (A) B cells purified from WT, TLR2-, TLR4-, or MyD88 deficient mice were stimulated with TRAPs (3 μg/mL) for 72 h. Frequency of IL-10-producing B cells was determined by flow cytometry. (B and C) B cells purified from WT mice were pre-treated with NF-κB inhibitor Bay11–7082 at different concentrations for 60 min, and then stimulated with TRAPs (3 μg/mL) for 72 h. Frequency of IL-10+ B cells was assessed by flow cytometry (B). IL-10 levels in supernatants were measured by ELISA (C). (D) B cells purified from WT, TLR2-, TLR4-, or MyD88 deficient mice were stimulated with TRAPs (3 μg/mL) for 1 h, and the expression of pNF-κB p65 was determined by flow cytometry. (E) CFSE-labeled CD4+ T or CD8+ T cells purified from WT mice were stimulated with plate-bound anti-CD3/anti-CD28 mAb and were either cultured alone (T cells only) or were co-cultured with TRAP-induced B cells (3 μg/mL) from WT, TLR2-, TLR4-, and MyD88 deficient mice at ratio of 1:1 for 4 d. T cell proliferation was evaluated by flow cytometry. Data (mean ± s.e.m) are representative of three independent experiments. ** p < 0.01, *** p < 0.001 by unpaired t-test.
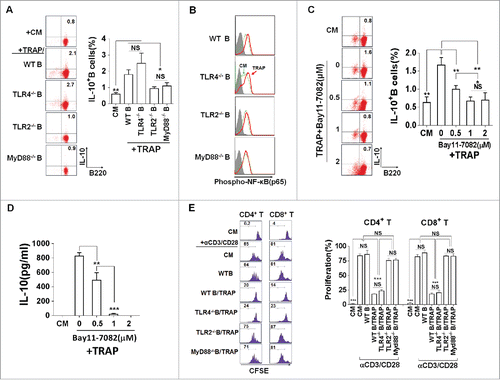
Figure 5. The expression of HMGB1 on TRAPs is essential for the induction of IL-10-producing B cells. (A) TRAPs derived from E.G7-OVA cells were labeled with anti-LC3 and anti-HMGB1 mAb, or control isotype. FACS analysis was then performed to determine HMGB1 level on the surface of LC3+ TRAPs. (B) TRAPs were pretreated with functional anti-HMGB1 mAb or control isotype overnight at 4°C and then used to stimulate purified B cells for 72 h. Frequency of IL-10+ B cells was assessed by flow cytometry. (C) The production of IL-10 by B cells was determined at 72 h after stimulated with TRAPs isolated from B16-F10 transfected with HMGB1-shRNA or Control-shRNA Plasmid. (D) TRAPs derived from WT B16-F10 cells were subjected to 20 cycles of sonication (30 sec sonication, 30 sec ice) and then used to stimulate purified B cells for 72 h. IL-10 production by B cells were determined by ELISA. Data (mean ± s.e.m) represent a typical result from three independent experiments. ** p < 0.01, *** p < 0.001 by unpaired t-test (C and D).
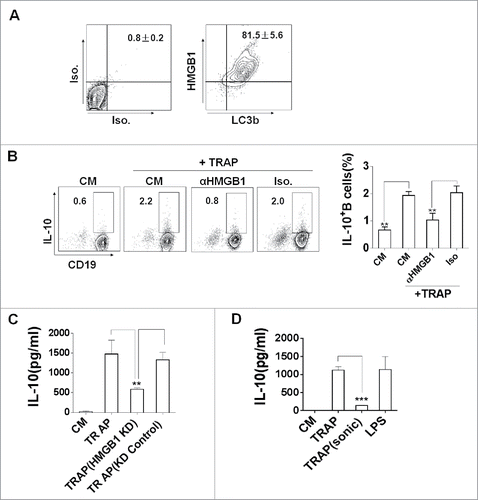
Figure 6. TRAPs from cancer patients induce human regulatory IL-10-producing B cells. (A and B) PBMC from four healthy donors was stimulated with TRAPs (1 μg/mL) from malignant effusions of 14 cancer patients or HepG2 cells for 72 h. The frequency of IL-10+ B cells was assessed by flow cytometry (A). ELISA determinations of IL-10 secretion in culture supernatants were also shown (B). (C) The expression of HMGB1 on human TRAPs was analyzed by flow cytometry, hTRAP1-3 were representative samples out of 14 cancer patients' TRAPs. hTRAP1-3 derived from patient 6, 7, and 2. (D) The frequencies of IL-10+ B cells induced by hTRAP1-3 were shown. (E) The graphs showed the correlation between intensities of HMGB1 expression in 14 cancer patients' TRAP and frequency of induced IL-10+ B cells. (F) TRAP-induced human B cells inhibit proliferation of T cells stimulated with anti-CD3/CD28 mAbs. Data (mean ± s.e.m) represent a typical result from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 by unpaired t-test (A and B) and Spearman's rank correlation (E).
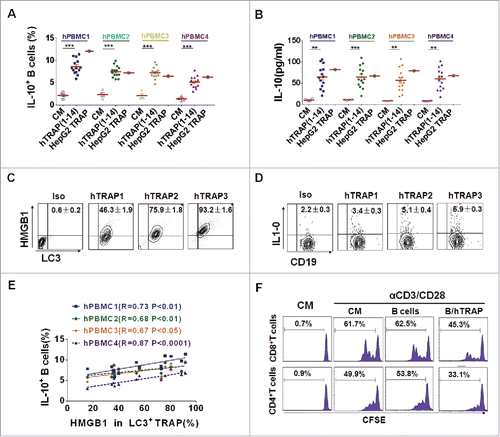
Figure 7. A proposed mode for the mechanisms and functions of IL-10-producing B cells induced by TRAPs. Membrane-bound HMGB1 on the intact TRAPs activate TLR2 on B cells, leading to TLR-mediated MyD88/NF-κB activation and secretion of IL-10 that may impair antitumor T cell response and ultimately lead to tumor growth.