Figures & data
Table 1. Patients' characteristics.
Figure 1. Study design. Vaccinations (V) consisted of three cycles (C1–C3) of four monthly subcutaneous (s.c.) injections of 0.5 mg of NY-ESO-179–108 long peptide. HLA-A2+ patients were also vaccinated with 0.1 mg of Melan-A26–35 native peptide and 20 μg of Mage-A10254–262 peptide in the first cycle, followed by 0.1 mg Melan-A26–35(A27L) analog peptide and 0.1 mg of Mage-A10254–262 peptide in the following cycles. In addition, Group B patients were treated with low dose rh-IL-2. Peptides for HLA-A2+ patients were emulsified in 1 mL Montanide® ISA-51 and 2 mg CpG-7909/PF-3512676, peptides for HLA-A2− patients were emulsified in 0.5 mL Montanide® ISA-51 and 1 mg CpG-7909/PF-3512676. The three vaccines of the cycle 3 were formulated without Montanide. Blood samples were withdrawn and PBMC were prepared at baseline (100 mL), after two vaccinations (two samples at 7 d interval: 30 and 100 mL) and after four vaccinations (two samples at 7 d interval: 30 and 100 mL) for the assessment of immune responses.
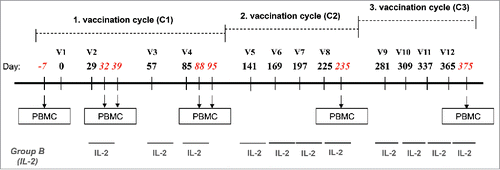
Figure 2. Specific CD8+ T-cell responses before and after vaccination with NY-ESO-1 LSP. (A) Representative example of a NY-ESO-1-specific CD8+ T-cell response 14 d after IVS. Cytokine secreting cells are enumerated after 6-hchallenging of the expanded cells with the NY-ESO-1 pool of overlapping peptides, or without any peptide as control. (B) Details of longitudinal NY-ESO-1-specific CD8+ T-cell responses (IFNγ, TNFα, and IL-2) measured individually in each patient before and during vaccination. (C) Polyfunctionality of NY-ESO-1-specific CD8+ T-cell responses assessed as IFNγ+TNFα+ or IFNγ+TNFα+IL-2+ cells, measured individually in each patient before and during vaccination. (D) Quantification of the contribution of each individual cytokine (IFNγ, TNFα, and IL-2) to the NY-ESO-1-specific CD8+ T-cell response, before and during vaccination. The mean of the response for each cytokine is shown for all patients grouped as % of the total response (that is defined as 100%). The magnitude (mean for all patients grouped) of the total response at each time point is indicated on the bottom of each pie.
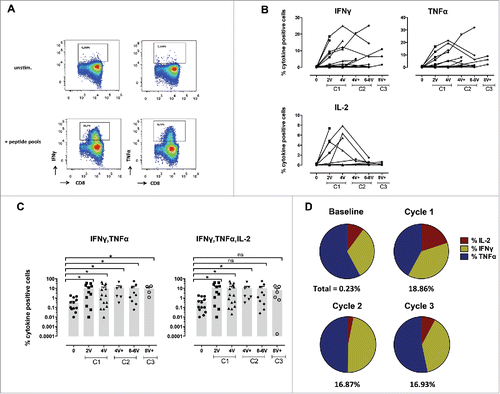
Figure 3. Specific CD4+ T-cell responses before and after vaccination with NY-ESO-1 LSP. (A) Representative example of a NY-ESO-1-specific CD4+ T-cell response 14 d after IVS. Cytokine secreting cells are enumerated after 6-h challenging of the expanded cells with the NY-ESO-1 pool of overlapping peptides, or without any peptide as control. (B) Details of longitudinal NY-ESO-1-specific CD4+ T-cell responses (IFNγ, TNFα, IL2, and IL-13) measured individually in each patient before and during vaccination. (C) Polyfunctionality of NY-ESO-1-specific CD4+ T-cell responses assessed as IFNγ+TNFα+, or IFNγ+TNFα+IL-2+, or IFNγ+TNFα+IL-2+IL-13+ cells, measured individually in each patient before and during vaccination. (D) Quantification of the contribution of each individual cytokine (IFNγ, TNFα, IL-13, and IL-2) to the NY-ESO-1-specific CD4+ T-cell responses, before and during vaccination. The mean of the response for each cytokine is shown for all patients grouped as % of the total response (that is defined as 100%). The magnitude (mean for all patients grouped) of the total response at each time point is indicated on the bottom of each pie.
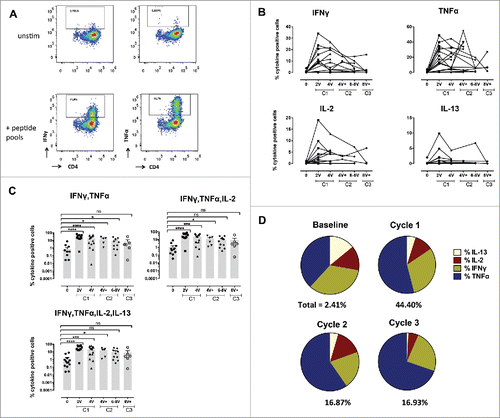
Figure 4. Summary of NY-ESO-1-specific CD8+ and CD4+ T-cell responses, and antibody responses. Cellular responses were measured 14 d after IVS and humoral responses were analyzed by ELISA against the NY-ESO-1 protein in plasma collected from enrolled patients' pre- treatment and during treatment as indicated.
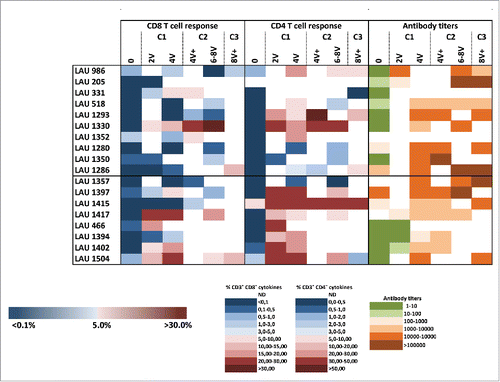
Figure 5. Mapping of NY-ESO-1-specific CD8+ and CD4+ T-cell responses. (A) Using individual overlapping peptides covering the entire NY-ESO-1 LSP sequence, NY-ESO-1-specific CD8+ T-cell responses (n = 5 patients) and CD4+ T-cell responses (n = 9 patients) were mapped, by monitoring IFNγ + TNFα (CD8+ T-cells) and IFNγ (CD4+ T-cells) production after 6-h peptide challenge. (B) Representative example of NY-ESO-194–104/B35 multimer staining directly ex vivo and after IVS of CD8+ T-cells from HLA-B35+ patients. (C) MHC class II restriction of NY-ESO-1-specific CD4+ T-cell responses was assessed in a 6-h peptide challenge in the absence or presence of blocking anti-DR, -DP, or -DQ antibodies. Specific responses were measured by quantification of IFNγ production. (D) Representative example of NY-ESO-187–99/DR7 multimer staining of IVS CD4+ T-cells obtained from HLA-DR7+ patients, before and during immunization.
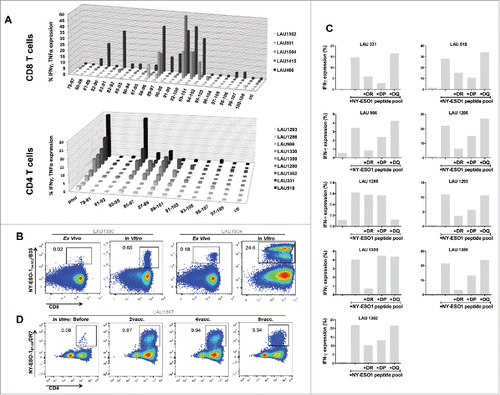
Table 2. Summary of frequencies of NY-ESO-1/DR7-specific CD4+ T-cells, detected after one round of IVS in HLA-DR7+ patients.
Figure 6. NY-ESO-187–99 peptide represents a novel MHC class II epitope. A. NY-ESO-187–99-specific CD4+ T-cell clones were generated and stained with NY-ESO-1/DR7 multimers (upper panels). Reactivity to specific peptide was tested and EC50 was calculated for both Type1 and Type2 cytokines (lower panels). (B) NY-ESO-187–99-specific-CD4+ T-cell clones were assessed for their capacity to secrete IFNγ or kill HLA-DR7+ target T-cells, in the presence or absence of specific peptide. (C) Representative example of direct ex vivo multimer staining of NY-ESO-187–99-specific-CD4+ T-cells in HLA-DR7+ patients. (D) Summary of frequencies of direct ex vivo detectable NY-ESO-187–99-specific-CD4+ T-cells in HLA-DR7+ patients.
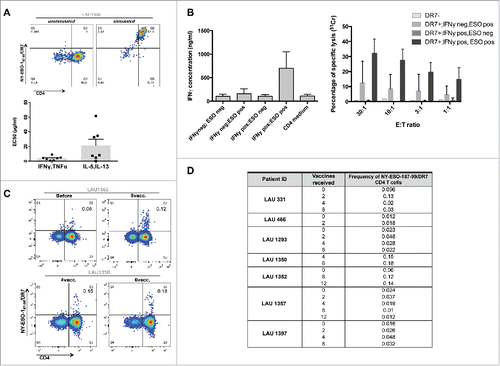
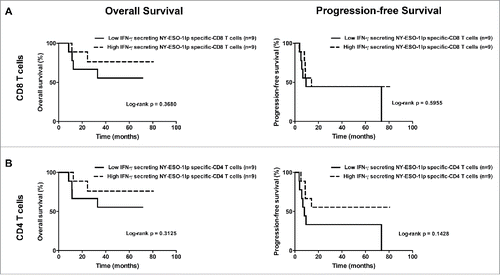
Figure 7. Overall survival and progression-free survival depending on the maximal level of IFNγ+ NY-ESO-1-specific CD8+ T-cell (A) and CD4+ T-cell (B) frequencies reached during the study after IVS. (A). Overall survival (left panel) and progression-free survival (right panel) in patients with low frequencies of IFNγ+ NY-ESO-1-specific CD8+ T-cells (lower than the median, n = 9) and in patients with high frequencies of IFNγ+ NY-ESO-1-specific CD8+ T-cells (higher than the median, n = 9). (B) Overall survival (left panel) and progression-free survival (right panel) in patients with low frequencies of IFNγ+ NY-ESO-1-specific CD4+ T-cells (lower than the median, n = 9) and in patients with high frequencies of IFNγ+ NY-ESO-1-specific CD4+ T-cells (higher than the median, n = 9).