Figures & data
Figure 1. γδ T cells in the LPL predominantly express Vγ6 and secrete IL-17 and are significantly increased in DSS-induced colon. (A) γδT cells in the LPL were stained with Vγ1, Vγ4, and Vγ6 mAbs and representative dot plots are shown. (B) LPLs were stimulated with PMA+ionomycin and intracellular IL-17 and IFNγ staining was performed. (C) Single cell suspensions from mLNs were stimulated with PMA+ionomycin and intracellular IL-17 and IFNγ staining was performed. Cells were gated on differential populations as indicated. (D) LPL from control and DSS-treated mice were stained with CD3, pan γδTCR, and intracellular IL-17. Total γδT cells and γδT17 cells were summarized. Each dot represents one mouse. (E) Groups of mice (n = 5) were treated with or without DSS water for indicated time and then killed. LPLs were stimulated with PMA+ionomycin and then stained with CD4 and γδ TCR mAbs and intracellular IL-17. Representative dot plots and summarized percent of Th17 and γδT17 cells are shown. *p < 0.05, **p < 0.01.
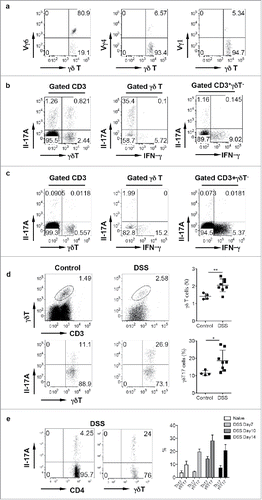
Figure 2. γδ T cells play a protective role in DSS-induced colitis. (A) WT and TCR δKO mice were treated with DSS water for 7 d. Colon tissues were collected for histological examination. Representative slides and accumulative scores are shown. (B) Colon tissues were put into Trizol and RNAs were extracted. The mRNA expression levels of IL-18, CXCL5, GM-CSF, and Arginase were measured by real-time PCR analysis. (C) Single cell suspensions from LPL of naïve WT and TCR δ KO mice were stained with CD11b and Gr-1 mAbs. (D) Single cell suspensions from LPLs of WT and TCR δ KO mice treated with DSS for 7 d were stained with CD11b and Gr-1 mAbs. Representative dot plots and summarized frequencies of Gr-1+CD11b+ cells are shown. (E) Groups of WT mice (n = 5) were treated with anti-IL-17, anti-IL-18, anti-CXCL5 mAb or isotype control mAb at days −2, 0, 2, and 5. Mice were fed with DSS water on day 0 for 7 d and then killed. LPLs were stained with Gr-1 and CD11b mAbs with viability dye. Representative dot plots and absolute numbers from each group are shown. (F) Splenocytes from OT-1 mice were labeled with CFSE and then co-cultured with Gr-1+CD11b+ cells sorted from LPL of DSS-treated WT mice at indicated ratios in the presence of OVA for 3 d. Cells were stimulated with PMA+ionomycin and intracellular IFNγ staining was performed. Cells were gated on CD8+ cells. Representative dot plots and summarized IFNγ-producing CD8 T cells are shown. *p < 0.05, **p < 0.01, ***p < 0.001.
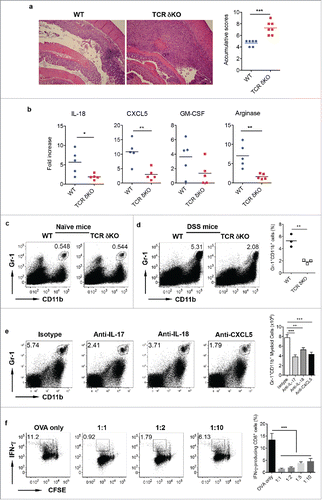
Figure 3. IFNγ-producing γδ T cells do not have a protective role in DSS-induced colitis. (A) CD27+ γδ T cells sorted from spleen and LNs were expanded ex vivo for 6 d. They predominately express CD27 and produce IFNγ but not IL-17. (B) Ex vivo expanded CD27+ γδ T cells were adoptively transferred into TCR δ KO mice. γδ T cell and CD27 staining in LPL, mLN, body LN, and spleen was shown. (C) Mice transferred with or without CD27+ γδ T cells were fed with DSS for 7 d. Representative histological slides and accumulative scores are shown. (D) Representative dot plots of Gr-1 and CD11b in LPL are shown. (E) Representative dot plots of Gr-1 and CD11b in mLN are shown.
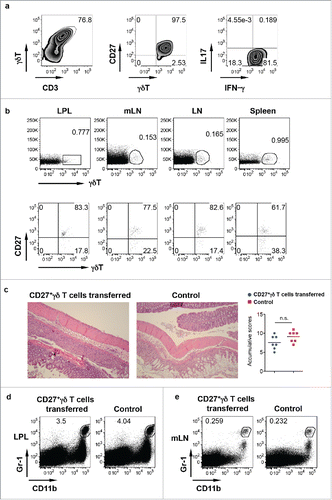
Figure 4. Mice reconstituted with γδT17 cells have milder colitis. (A and B) Neonatal thymocytes from WT mice were transferred into lethally irradiated TCR δKO mice following BM cell transfer from TCR δ KO mice. Mice were reconstituted for at least eight weeks. Mice with BM cell transfer alone were used as control. γδT cell and intracellular IL-17 and IFNγ staining was performed in mLN (A) and LPL (B), respectively. (C) Reconstituted mice were fed with DSS water for 7 d. Representative histological slides and accumulative scores are shown. (D) Representative dot plots of Gr-1 and CD11b staining in LPL are shown. (E) Splenocytes from OT-1 mice were labeled with CFSE and then co-cultured with γδ T cells sorted from LPL of DSS-treated WT mice (day 7) in the presence of OVA for 3 d. Cells were stimulated with PMA+ionomycin and intracellular IFNγ staining was performed. Cells were gated on CD8+ cells. Representative dot plots and summarized IFNγ-producing CD8 T cells are shown. (F) LPLs from DSS-treated mice were stained with CD3, CD4, γδ TCR, and intracellular galectin-1 and galectin-9. Summarized percentages of galctin-1 or galectin-9-positive CD4 or γδ T cells are shown. *p < 0.05, **p < 0.01.
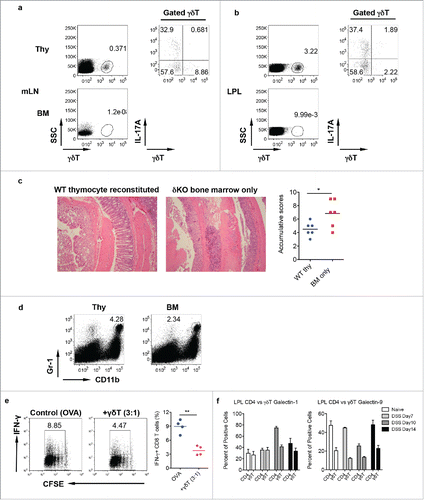
Figure 5. Rictor-deficiency significantly reduces γδT17 cells and abrogates γδT-cell mediated protective activity in DSS-induced colitis. (A) γδT cell staining in LPL of control and Rictor cKO mice. (B) Single cell suspensions from LP were stimulated with PMA+ionomycin and intracellular IL-17 staining was performed. Representative dot plots and summarized γδT17 percentages are shown. (C) Mice reconstituted with neonatal thymocytes from Rictor control or cKO mice were fed with DSS water for 7 d. Representative histological slides and accumulative scores are shown. (D) Representative dot plots of Gr-1 and CD11b staining and summarized Gr-1+CD11b+ myeloid cells in LPL of DSS-treated mice are shown. *p < 0.05, ***p < 0.001.
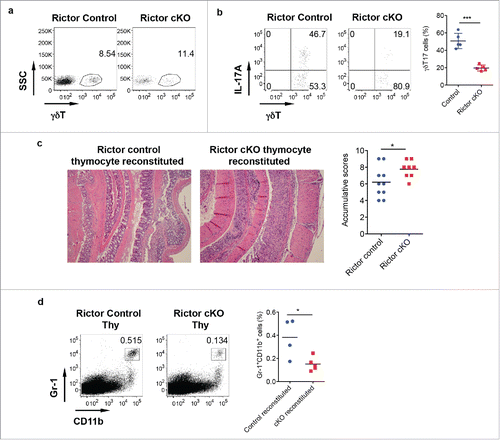
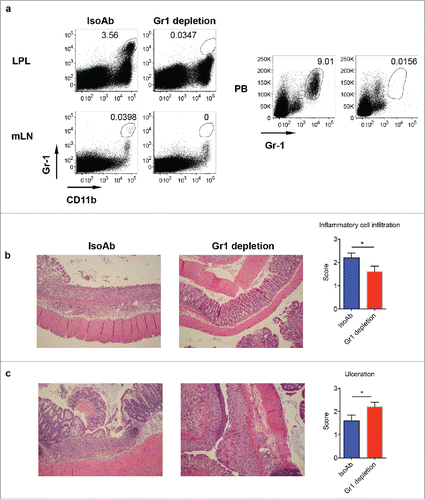
Figure 6. Depletion of Gr-1+CD11b+ myeloid cells induces severe disease in DSS-induced colitis. (A) WT mice were treated with anti-Gr-1 or isotype control mAb. Representative dot plots show that anti-Gr-1 effectively depleted this subset from LPL, mLN and periphery. (B) Mice injected with Gr-1 or isotype mAb were fed with DSS water for 7 d. Representative histological slides show less inflammatory cell infiltration in Gr-1 depleted mice. Summarized inflammatory cell infiltration scores are shown. (C) Representative histological slides show severe mucosal erosion and ulceration in Gr-1 depleted mice. Summarized ulceration scores are shown. *p < 0.05.