Figures & data
Table 1. Clinical-pathological features of melanoma patients treated with immune checkpoint blockade agents or with standard/targeted therapies.
Figure 1. sNKG2DLs in the serum of melanoma patients treated with immune checkpoint blockade agents. The presence of sMICA (Panel A), sMICB (Panel B), and sULBP-2, 3 (Panels D and E) in the serum at baseline (W1; black circle) and post-treatment (W12; black square) of melanoma patients (N = 162) treated with immune checkpoint blockade agents was measured by ELISA assay (see Material and methods). ULBP-1 determinations at pre- and post-treatment were performed in N = 131 and 128 patients, respectively (Panel C). Mean and error bars are shown in the graphs. As negative control the serum of N = 10 HD was used in ELISA assays (data not shown; see Ref. Citation[25]).
![Figure 1. sNKG2DLs in the serum of melanoma patients treated with immune checkpoint blockade agents. The presence of sMICA (Panel A), sMICB (Panel B), and sULBP-2, 3 (Panels D and E) in the serum at baseline (W1; black circle) and post-treatment (W12; black square) of melanoma patients (N = 162) treated with immune checkpoint blockade agents was measured by ELISA assay (see Material and methods). ULBP-1 determinations at pre- and post-treatment were performed in N = 131 and 128 patients, respectively (Panel C). Mean and error bars are shown in the graphs. As negative control the serum of N = 10 HD was used in ELISA assays (data not shown; see Ref. Citation[25]).](/cms/asset/7a240c02-91d1-4d36-a48d-f5e2ffb48a33/koni_a_1323618_f0001_b.gif)
Figure 2. Detection of sNKG2DLs in the serum of control group melanoma patients. The presence of soluble NKG2DLs (MICA, Panel A; MICB, Panel B; ULBP-1, Panel C; ULBP-2, Panel D; ULBP-3, Panel E) was measured by ELISA assay (see Material and methods) in the baseline serum (W1; black circle) of melanoma patients (N = 65) treated with either standard or BRAFi therapies. Mean and error bars are shown in the graphs. As negative control the serum of N = 10 HD was used in ELISA assays (data not shown; see Ref. Citation[25]).
![Figure 2. Detection of sNKG2DLs in the serum of control group melanoma patients. The presence of soluble NKG2DLs (MICA, Panel A; MICB, Panel B; ULBP-1, Panel C; ULBP-2, Panel D; ULBP-3, Panel E) was measured by ELISA assay (see Material and methods) in the baseline serum (W1; black circle) of melanoma patients (N = 65) treated with either standard or BRAFi therapies. Mean and error bars are shown in the graphs. As negative control the serum of N = 10 HD was used in ELISA assays (data not shown; see Ref. Citation[25]).](/cms/asset/f821851d-964e-4bde-b295-3b309b846d85/koni_a_1323618_f0002_b.gif)
Table 2. Association between the levels at baseline serum of sNKG2DLs and the disease control of melanoma patients.
Table 3. Association between the levels at baseline serum of sNKG2DLs and the OS of melanoma patients.
Figure 3. Overall survival of melanoma patients treated with immunotherapy in relation with the presence or not of sNKG2DLs in serum. Kaplan–Meier plots of overall survival of melanoma patients treated with immune checkpoint blockade agents (Panels A and B) or with standard or BRAFi therapies (Panels C and D) in relation with the detection (black line) or not (dotted line) at baseline of sMICB (Panels A and C) and sULBP-1 (Panels B and D). The baseline serum levels of sMICB (Panel A) and ULBP-1 (Panel B) could discriminate melanoma patients with long-term survival (median OS = 21.6 and 25.3 mo, p = 0.02 and 0.01, respectively) from poor survivors (median OS = 8.8 and 12.1 mo, respectively) for the cohort of patients treated with immune checkpoint blockade agents. Panels C and D show the absence of association between the serum levels of these ligands and OS in the control group of patients (median OS = 12.0 vs. 13.1 and 8.5 vs.15.6 and p = 0.4 and 0.85, respectively).
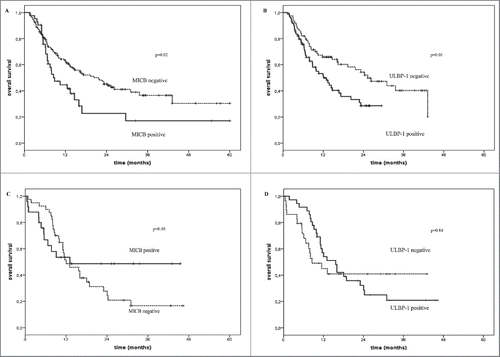
Figure 4. Overall survival of melanoma patients treated with immunotherapy in association with the levels of sNKG2DLs in post-treatment serum. The absence (dotted line) in the post-treatment serum of sMICA (Panel A) and sMICB (Panel B) correlated with improved OS (median OS = 20.2 and 22.8 vs. 10.4 mo, p = 0.02 and 0.01, respectively) while detectable levels of these molecules were found in the serum of poor survivor patients (median OS = 12.4 and 10.4 mo, respectively) (black line).
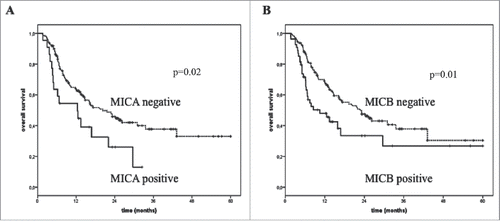
Table 4. COX regression analysis to identify biomarkers influencing the clinical outcome of melanoma patients.
