Figures & data
Figure 1. Study design and CONSORT diagram of patient flow. (A) Study design for vaccination and evaluation. CR, complete remission; Pts, patients; SR, surgical recession; RFA, radiofrequency ablation; PEI, percutaneous ethanol injection; TACE, transarterial chemoembolization; ICF, Informed consent form; LKP, leukaperisis; DC, dendritic cell; RFS, recurrence-free survival; CT, computed tomography; MRI, magnetic resonance imaging; TTR, time-to-recurrence; mOS, median overall survival. (B) Patient flow from random assignment *excluded according to ITT analysis-based criteria. Of the enrolled patients, 23 did not meet inclusion criteria and thus were excluded from random assignment. In addition, 12 more patients were excluded from the efficacy analysis because they were found to violate inclusion criteria after randomization.
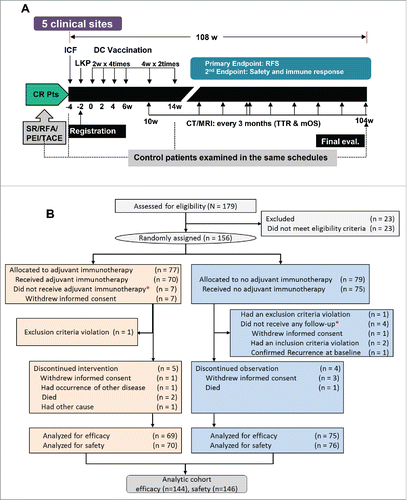
Table 1. Baseline demographics and disease characteristics.
Figure 2. Kaplan–Meier estimates of recurrence-free survival (RFS) in (A) overall patients, (B) patients of non-RFA group, (C) patients of RFA group, (D) patients of surgical resection group and (E) patients of surgical resection group (PP analysis), (F) Kaplan–Meier estimates of RFS in patients of TACE group, (G) Kaplan-Meier estimates of RFS in non-RFA group patients with stage 2 and 3 diseases. RFS was computed on all patients included in the ITT population (except E). Patients who had not progressed or died were censored on data cut-off. p values calculated by log-rank test (and by Wilcoxon test). Note: HR, hazard ratio; CI, confidence interval.
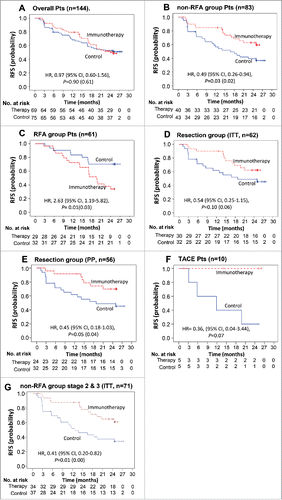
Table 2. Subgroup analysis of recurrence rates between control and immunotherapy groups over 2 y.
Figure 3. Overall survival Kaplan–Meier estimates of OS in overall patients. OS was computed on all patients included in the ITT population.
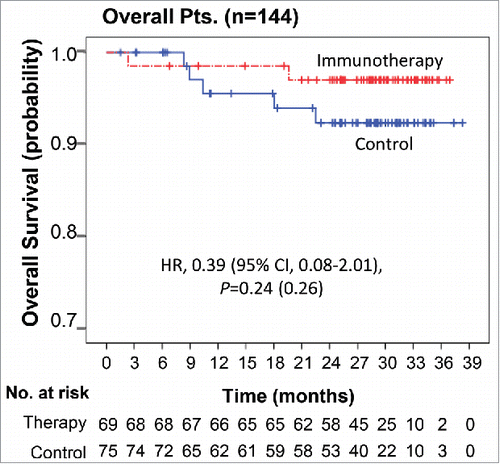
Figure 4. Immune responses after DC vaccination Changes in immune responses before and after DC vaccination against a tumor-associated antigen mixture (AFP, GPC-3, and MAGE-1). Data are represented after subtraction with the count of no Ag. (A) IFNγ ELISPOT assays were performed with PBMCs obtained from each patient at the indicated time points after the start of DC vaccination (0 w). Analysis was performed by paired t-test (GraphPad PRISM, ver 6.07) with the indicated number of patients. (B) Antigen-specific lymphocyte proliferation assays were performed after DC vaccinations using autologous PBMCs at each time point. Proliferation was determined by 3H-thymidine incorporation (DPM, disintegrations per minute) using a liquid scintillation counter and the results were analyzed using the same method as for the ELISPOT assay. **p < 0.01, ***p < 0.001, ****p < 0.0001.
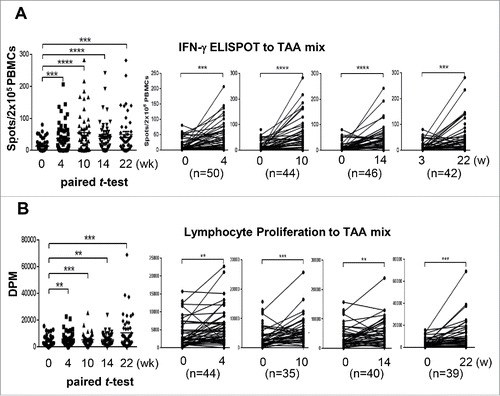
Table 3. Treatment-emergent adverse events (safety population).
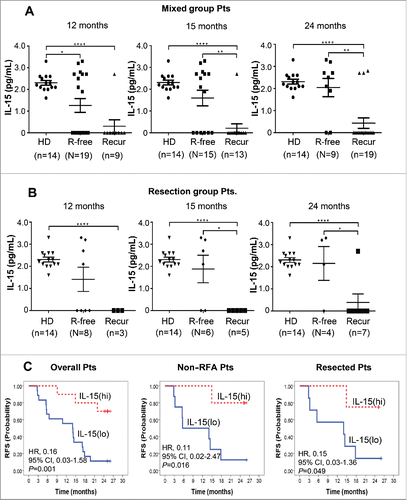
Figure 5. Baseline IL-15 level in the sera of healthy donors (HD) and patients with (Recur) or without (R-free) tumor-recurrence at the indicated time points after DC vaccination in mixed group patients (A) and resected patients (B). IL-15 levels were assessed by Luminex Bead-based Milliplex#x08E8; Multiplex Assay system (Millipore, St Charles, MO, USA). Assay sensitivity of the system; minimum detectable concentration was 0.6 pg/mL at a short protocol (n = 4 assays). Precision; intra-assay % coefficient of variance (CV) was 6.7 and extra-assay %CV was 9.5. Accuracy; 100.5 at spike recovery in serum matrix (6 Point Spikes). Statistical analysis was performed by unpaired t-test with the number (n) of patients: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (C) Baseline IL-15 level and RFS after DC immunotherapy. Twenty-eight patients from the DC vaccination group were assessed. IL-15(hi) and IL-15(lo) represent patients with baseline IL-15 >0.6 pg/mL (higher than minimal detectable concentration) and baseline IL-15<0.6 pg/mL (lower than minimal detectable concentration), respectively. HRs and p values were represented.