Figures & data
Figure 1. Determination of specificity for IL-5Rα staining by immunohistochemistry (IHC). (A) IL-5Rα-negative and -positive adipose and placental tissue, respectively, were stained in the presence of the IL-5Rα-specific polyclonal antibody. (B) IL-5Rα-positive tissue was stained with the IL-5Rα-specific polyclonal antibody in the presence of 100-fold excess blocking peptide. (C) IL-5Rα staining of bladder cancer specimens is predominantly paranuclear, cytoplasmic, and plasma membrane, respectively (arrow), (arrowhead), and (dashed arrow).
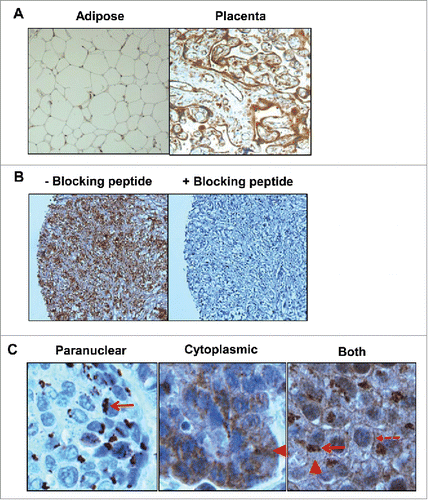
Table 1. IL-5Rα expression in bladder cancer.
Figure 2. IL-5Rα expression in normal and bladder cancer primary specimens at various pathology stages by IHC. Representative examples from individual patients of the increase in IL-5Rα protein expression levels in healthy versus cancer specimens. (A) One patient whose bladder contained normal urothelium and tumors with TNM stages pT1, pT2, and pT3. In the pT1 specimen, IL-5Rα-positive nests are observed (red arrow) within the lamina propria. In the pT2 and pT3 specimens, there is dissemination of IL-5Rα-positive tumor cells in the lamina propria and fat, respectively. (B) Another patient whose bladder contained normal urothelium versus pT2 tumor tissue. (C) Patient with tumor specimens obtained at initial visit and follow-up visit for tumor recurrence. Images are at ×40 magnification except for image of pT3 tumor in A, which is at ×400 magnification.
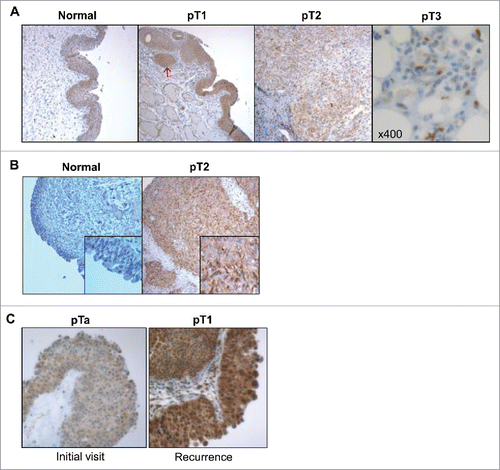
Table 2. Bladder tumor recurrence and IL-5Rα expression.
Figure 3. In vivo model of human IL-5Rα-positive MIBC. IL-5Rα staining by IHC on HT-1376 and HT-B9 heterotopic xenografts grown in NOD/SCID mice. Staining of xenografts was processed in identical fashion to human tumor specimens.
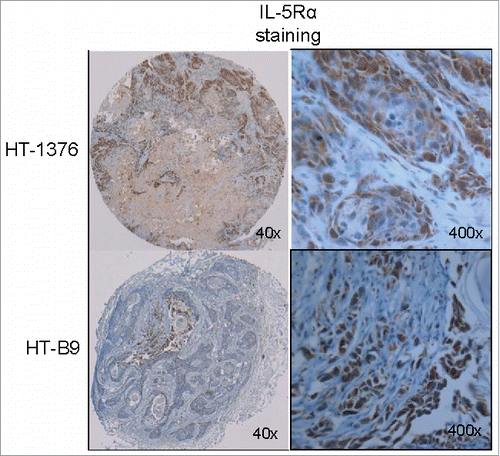
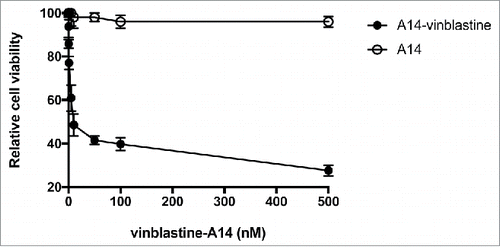
Figure 4. Saturation binding of HT-1376 and HT-B9 cells with 64Cu-A14. Specific binding curves for 64Cu-A14 on HT-1376 and HT-B9 cells. Specific binding is plotted in decay-corrected counts per minute (CPM) versus increasing 64Cu-A14 concentration (nM).
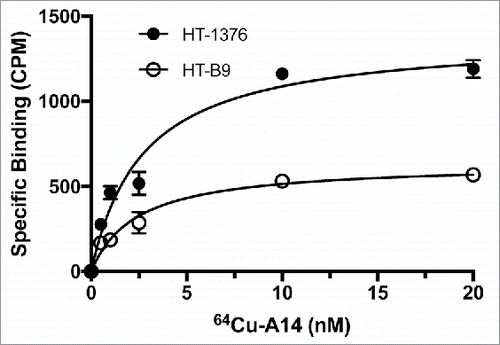
Figure 5. In vivo PET imaging of HT-1376 and HT-B9 tumors by 64Cu-A14. 48 h post-injection representative PET images of tumor-bearing NOD/SCID mice intravenously injected with 64Cu-A14 with and without A14 pre-dosing to block IL-5Rα sites. White arrows indicate HT-1376 tumors, white arrowheads indicate HT-B9 tumors, and green arrows indicate the liver, which has the highest uptake due to normal antibody metabolism. The reductions in the tumor uptakes in A14-pre-dosed mice are statistically significant (p < 0.05).
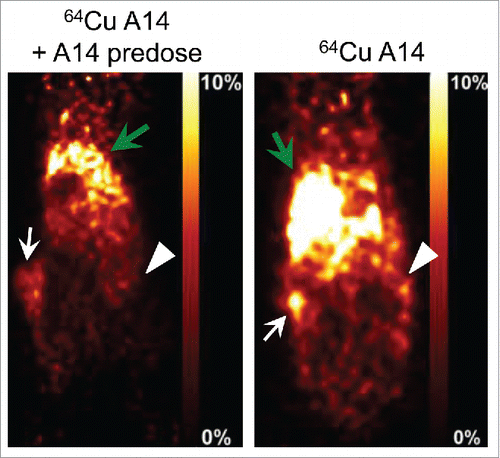
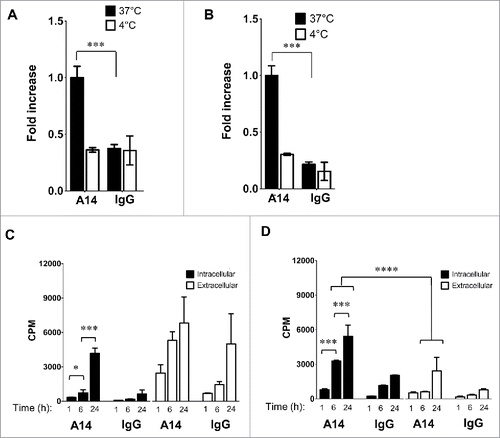
Figure 6. IL-5Rα cellular distribution and expression dynamics when stimulated with IL-5. Confocal microscopy analysis of IL-5Rα-specific immunofluorescence in HT-1376 cells after treatment with IL-5 at increasing time points at either 4°C or 37°C.
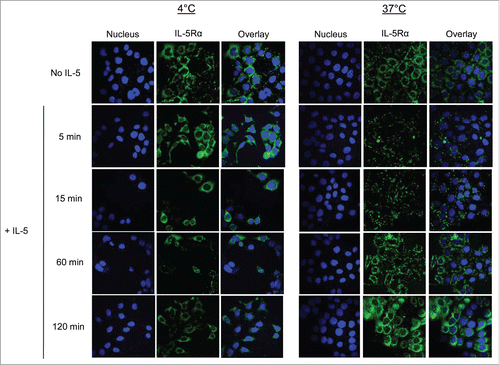
Figure 7. Internalization and intracellular radioactivity accumulation in MIBC cells after treatment with 64Cu-A14. The relative radioactivity intracellular accumulation increase in HT-1376 (A and C) and HT-B9 (B and D) cells after treatment with 64Cu-A14 and 64Cu-IgG at 1 h at 37°C compared with 4°C and when treated under increasing incubation times. p-values of < 0.05, <0.001, and <0.0001 are indicated by *, **, ***, respectively. For clarity, p-values are focused on the favorable 64Cu-A14-specific increase of intracellular radioactivity accumulation over time and the retention of intracellular versus extracellular radioactivity.