Figures & data
Figure 1. Mechanisms leading to transitory or sustained PD-1 expression in activated and exhausted T cells. Left panel: Upon TCR-mediated stimulation, NFAT is dephosphorylated and translocated into the nucleus, where, upon association with AP-1 complex activated upon CD28 signaling, it drives effector gene and PD-1 expression. Right panel: In the context of chronic antigen stimulation, sustained TCR signaling leads to a continuous PD-1 expression. Upon PD-L1 ligation, induced by IFN-γ in the microenvironment, PD-1 pathway inhibits TCR and CD28 signaling, that decreases AP-1 activation. Once translocated into the nucleus, NFAT is mainly “partnerless” and drives exhaustion genes and a constant PD-1 expression, facilitated by a constitutively demethylated PDCD1 promoter.
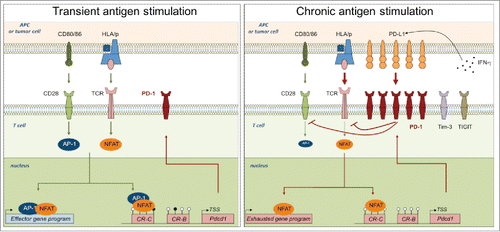
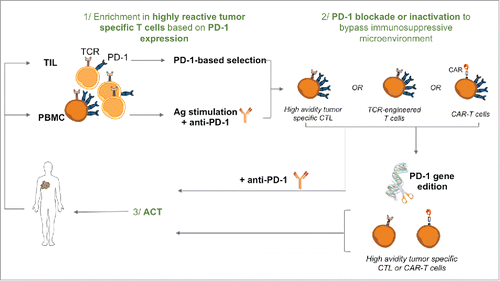
Figure 2. PD-1 based selection and inactivation for optimal T-cell based therapies. 1/ High avidity tumor specific T cells can be expanded from the CD8+ PD-1+ TIL fraction or after antigen stimulation of PBMC upon PD-1 blockade that favors the amplification of highly reactive CD8+ T cells. 2/ PD-1+ recovered high avidity T cells, CAR-T cells or TCR-transduced T cells can be further inactivated for PD-1 expression (and other inhibitory receptors) by genome editing, to bypass immunosuppression mechanisms. 3/ High avidity tumor specific T cells inactivated for PD-1 expression can be infused to cancer patients, alone or in combination with other therapies such as radioimmunotherapy.