Figures & data
Figure 1. Phenotype and function of CD8+ and CD4+ T cells in lymphoma-bearing Eμ-myc mice. (A) Frequencies and (B) absolute numbers of CD8+ and CD4+ T cells in spleens from BL/6 mice, non-lymphoma-bearing (Eµ-myc-L) and lymphoma-bearing Eµ-myc mice (Eµ-myc+L) (n = 7–10 mice/group). (C) Expression and mean fluorescence intensities (MFI) of indicated inhibitory receptors on CD8+ T cells from spleens of BL/6 mice, Eµ-myc-L and Eµ-myc+L mice determined by flow-cytometry (black: staining, gray: isotype, MFI: MFI staining – MFI isotype). One representative FACS plot out of 7–12 is shown. (D) Frequency of co-expression of PD-1, 2B4 and LAG-3 on splenic CD8+ and CD4+ T cells from BL/6 (blue), Eµ-myc-L (green) and Eµ-myc+L (red) mice (n = 5–7 mice/group). (E) Eµ-myc mice were crossed to lymphocytic choriomeningitis virus (LCMV) gp33 TCR transgenic p14 mice (Eµ-myc x p14) and frequency of co-expression of inhibitory receptors on p14pos T cells was analyzed in non-lymphoma bearing Eµ-myc x p14–L (blue) and lymphoma bearing Eµ-myc x p14+L (orange) mice. (n = 2–3 mice/ group). (F) Frequency of PD-1, 2B4, and LAG-3 expressing CD8+ (upper panel) and CD4+ T cells (lower panel) were determined and correlation with absolute B cell numbers was analyzed (n = 6 mice/group). Data are depicted as linear regression curves, calculated using Ozone correlations (Graph Pad Prism5). The “goodness of fit” is displayed as the coefficient of determination ( = r2) (perfect fit: r2 = 1) and significance is displayed as p values (p < 0.05, p < 0.01, p < 0.0001). The correlation coefficient r was calculated using Pearson correlation (perfect correlation: r = 1). (G) CD8+ and (H) CD4+ T cells were isolated from spleens of BL/6, Eµ-myc-L and Eµ-myc+L (n = 25–22 mice/group for CD8+; n = 3–9 mice/group for CD4+) and 3[H]-TdR incorporation was assessed after stimulation with plate-bound αCD3 monoclonal antibody (mAb) for 72 h. (I) Annexin-V staining of splenic CD8+ or CD4+ T cells, from BL/6 and Eµ-myc+L mice directly ex vivo (black: staining, gray: isotype). One representative plot out of 4 is shown. (J) Frequency of Annexin-V+ CD8+ and CD4+ T cells from BL/6 and Eµ-myc+L mice (n = 4 mice/group). Data are displayed as mean ± SEM. Statistics: Student's t test. *p < 0.05, ***p < 0.0001. See also Supplementary Figure S1.
![Figure 1. Phenotype and function of CD8+ and CD4+ T cells in lymphoma-bearing Eμ-myc mice. (A) Frequencies and (B) absolute numbers of CD8+ and CD4+ T cells in spleens from BL/6 mice, non-lymphoma-bearing (Eµ-myc-L) and lymphoma-bearing Eµ-myc mice (Eµ-myc+L) (n = 7–10 mice/group). (C) Expression and mean fluorescence intensities (MFI) of indicated inhibitory receptors on CD8+ T cells from spleens of BL/6 mice, Eµ-myc-L and Eµ-myc+L mice determined by flow-cytometry (black: staining, gray: isotype, MFI: MFI staining – MFI isotype). One representative FACS plot out of 7–12 is shown. (D) Frequency of co-expression of PD-1, 2B4 and LAG-3 on splenic CD8+ and CD4+ T cells from BL/6 (blue), Eµ-myc-L (green) and Eµ-myc+L (red) mice (n = 5–7 mice/group). (E) Eµ-myc mice were crossed to lymphocytic choriomeningitis virus (LCMV) gp33 TCR transgenic p14 mice (Eµ-myc x p14) and frequency of co-expression of inhibitory receptors on p14pos T cells was analyzed in non-lymphoma bearing Eµ-myc x p14–L (blue) and lymphoma bearing Eµ-myc x p14+L (orange) mice. (n = 2–3 mice/ group). (F) Frequency of PD-1, 2B4, and LAG-3 expressing CD8+ (upper panel) and CD4+ T cells (lower panel) were determined and correlation with absolute B cell numbers was analyzed (n = 6 mice/group). Data are depicted as linear regression curves, calculated using Ozone correlations (Graph Pad Prism5). The “goodness of fit” is displayed as the coefficient of determination ( = r2) (perfect fit: r2 = 1) and significance is displayed as p values (p < 0.05, p < 0.01, p < 0.0001). The correlation coefficient r was calculated using Pearson correlation (perfect correlation: r = 1). (G) CD8+ and (H) CD4+ T cells were isolated from spleens of BL/6, Eµ-myc-L and Eµ-myc+L (n = 25–22 mice/group for CD8+; n = 3–9 mice/group for CD4+) and 3[H]-TdR incorporation was assessed after stimulation with plate-bound αCD3 monoclonal antibody (mAb) for 72 h. (I) Annexin-V staining of splenic CD8+ or CD4+ T cells, from BL/6 and Eµ-myc+L mice directly ex vivo (black: staining, gray: isotype). One representative plot out of 4 is shown. (J) Frequency of Annexin-V+ CD8+ and CD4+ T cells from BL/6 and Eµ-myc+L mice (n = 4 mice/group). Data are displayed as mean ± SEM. Statistics: Student's t test. *p < 0.05, ***p < 0.0001. See also Supplementary Figure S1.](/cms/asset/b6b3cc72-94de-4e0a-8511-6635cd4b5f5b/koni_a_1365997_f0001_oc.gif)
Figure 2. Impaired CD8+ T cell function in lymphoma-bearing mice after immunization with dendritic cells (DCs) or LCMV. (A-H) BL/6, Eµ-myc-L and Eµ-myc+L mice were immunized day 0 and day 2 i.v. with 2 × 105 H8 DCs and CD8+ T cells were analyzed in spleen 8 d after primary immunization. (A, B, E) Frequencies, (C, F) absolute numbers and (D, G) MFI index of cytokine expressing CD8+ T cells from BL/6, Eµ-myc-L and Eµ-myc+L mice after 5 h of in vitro re-stimulation with LCMV gp-33 are shown (n = 5 mice/group). (H) 3 × 107 gp33-pulsed CFSElo labeled B cells and unpulsed CFSEhi labeled B cells were injected into H8 DC-immunized BL/6 and Eµ-myc+L mice (n = 3–6 mice/group). Naïve BL/6 and Eµ-myc+L mice served as controls (n = 2 mice/group). In vivo cytolytic activity of CD8+ T cells was determined by measuring antigen-specific elimination of gp33-pulsed B cells 1 and 4 h after transfer by flow-cytometry. (I-P) BL/6 and Eµ-myc+L mice were infected with 200 pfu LCMV-WE i.v. CD8+ and CD4+ T cells were analyzed in the spleen 8 d after infection. (I, J) Frequencies, (K) absolute numbers and (L) MFI indexes of IFN-γ and TNF-α positive CD8+ T cells after re-stimulation with LCMV gp33 in vitro (n = 4 mice/group). (M) LCMV titres in livers and spleens (n = 4 mice/group). Dotted line indicated detection limit of the assay. (N) Frequencies, (O) absolute numbers and (P) MFI indexes of IFN-γ and TNF-α positive CD4+ T cells after re-stimulation with LCMV gp13 in vitro (n = 4 mice/group). For (A)and I, one representative dot plot out of 5 is depicted and frequencies are displayed as Mean ± SEM (n = 5 mice/group). Expression values for D, G, L, and (P) are displayed as MFI Index ( = intensity of expression in cytokine positive / cytokine negative cells). Data are displayed as mean ± SEM. Statistics: (B-G) One-way ANOVA, (H, J-P) Student's t test. *p < 0.05, **p < 0.01, ***p < 0.0001.
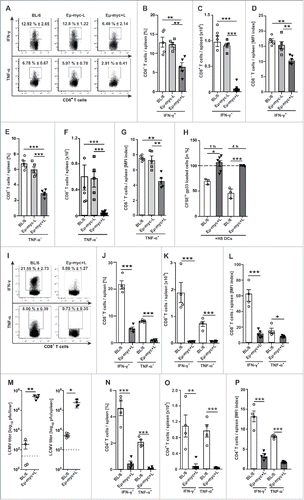
Figure 3. Dysfunctional p14 CTLs from lymphoma-bearing mice regain effector function after adoptive transfer into healthy recipient mice. (A-D) 1 × 106 congenic CD45.1+ p14 T cells were adoptively transferred into lymphoma-bearing Eµ-myc or BL/6 mice (n = 5–8 mice/group). Mice were immunized twice with 2 × 105 H8-DCs (day 0 and day 2). (A, B) Frequency, (C) total numbers and (D) MFI index of IFN-γ− and TNF-α-producing p14 CTLs in the spleen 6 d after immunisation. (E-H) 1 × 106 p14 CTLs were adoptively transferred into Eµ-myc+L or BL/6 mice (n = 9 mice/group). 18 h later p14 CTLs were activated with 1 × 104 pfu LCMV. (E, F) Frequency, (G) total number and (H) MFI index of IFN-γ and TNF-α-producing p14 CTLs in the spleen after re-stimulation in vitro with gp33. One representative dot plot out of 4–5 is depicted. (I) MACS-purified p14 CTLs from Eµ-myc x p14-L and +L mice were isolated and re-stimulated in vitro with gp33-pulsed irradiated splenocytes for 72 h and 3[H]-TdR incorporation of p14 CTLs was measured. (J-M) 1 × 106 MACS-purified p14 CTLs from Eµ-myc x p14-L and +L mice were adoptively transferred i.v. in naive BL/6 recipients and activated 18 h later with 1 × 104 pfu LCMV i.v.. (J) Total number, (K) frequencies (L) absolute numbers and (M) MFI index of IFN-γ and TNF-α producing p14 CTLs after re-stimulation with gp33 in vitro were assessed in the spleen 6 d after LCMV infection (n = 9 mice/group). For A, E, and K one representative dot plot out of 5 or 9 is depicted and frequencies are displayed as mean ± SEM (n = 5 (A); n = 9 (E, K) mice/group). Expression values for D, (H) and, M are displayed as MFI index ( = intensity of expression in cytokine positive / cytokine negative cells). Data are displayed as mean ± SEM. Statistics: Student's t test. *p < 0.05, **p < 0.01, ***p < 0.0001.
![Figure 3. Dysfunctional p14 CTLs from lymphoma-bearing mice regain effector function after adoptive transfer into healthy recipient mice. (A-D) 1 × 106 congenic CD45.1+ p14 T cells were adoptively transferred into lymphoma-bearing Eµ-myc or BL/6 mice (n = 5–8 mice/group). Mice were immunized twice with 2 × 105 H8-DCs (day 0 and day 2). (A, B) Frequency, (C) total numbers and (D) MFI index of IFN-γ− and TNF-α-producing p14 CTLs in the spleen 6 d after immunisation. (E-H) 1 × 106 p14 CTLs were adoptively transferred into Eµ-myc+L or BL/6 mice (n = 9 mice/group). 18 h later p14 CTLs were activated with 1 × 104 pfu LCMV. (E, F) Frequency, (G) total number and (H) MFI index of IFN-γ and TNF-α-producing p14 CTLs in the spleen after re-stimulation in vitro with gp33. One representative dot plot out of 4–5 is depicted. (I) MACS-purified p14 CTLs from Eµ-myc x p14-L and +L mice were isolated and re-stimulated in vitro with gp33-pulsed irradiated splenocytes for 72 h and 3[H]-TdR incorporation of p14 CTLs was measured. (J-M) 1 × 106 MACS-purified p14 CTLs from Eµ-myc x p14-L and +L mice were adoptively transferred i.v. in naive BL/6 recipients and activated 18 h later with 1 × 104 pfu LCMV i.v.. (J) Total number, (K) frequencies (L) absolute numbers and (M) MFI index of IFN-γ and TNF-α producing p14 CTLs after re-stimulation with gp33 in vitro were assessed in the spleen 6 d after LCMV infection (n = 9 mice/group). For A, E, and K one representative dot plot out of 5 or 9 is depicted and frequencies are displayed as mean ± SEM (n = 5 (A); n = 9 (E, K) mice/group). Expression values for D, (H) and, M are displayed as MFI index ( = intensity of expression in cytokine positive / cytokine negative cells). Data are displayed as mean ± SEM. Statistics: Student's t test. *p < 0.05, **p < 0.01, ***p < 0.0001.](/cms/asset/52badbe6-fa6b-4844-9f67-305219438381/koni_a_1365997_f0003_oc.gif)
Figure 4. Blockade of PD-1 signaling partially restores T cell function. (A) PDL-1 expression was analyzed on CD19+, CD11b+ or CD11c+ cells on splenocytes from BL/6 or Eµ-myc+L mice by flow-cytometry. One representative histogram out of 3 is shown. (black: staining, gray: isotype; n = 3 mice/group) (B) MACS-purified CD8+ T cells from naive BL/6 mice (responders) were activated with plate-bound αCD3 mAb (2 µg/ml) in the presence of titrated numbers of MACS-purified CD19+ B cells from naïve BL/6 or Eµ-myc+L mice for 72 h in triplicate cultures. 3[H]–TdR was added to the culture for the last 18 h of incubation. 1 representative out of 12 experiments is shown. (C) CD8+ T cells were cultured with titrated amounts of MACS-purified CD19+ B cells from Eµ-myc+L in the absence or presence of a blocking αPD-1 mAb (10 μg/ml) or control IgG and proliferation was measured as described in (B). Naïve BL/6 CD8+ T cells were cultured in the presence or absence of a αPD-1 or isotype control mAb served as controls. (D) Titrated numbers of MACS purified, irradiated (1000 rad) CD11b+ and CD11c+ APCs were cultured with CD8+ T cell responders (as described in (B) in the presence or absence (hatched bars) of an αPD-1 mAb (10 μg/ml) or control IgG and 3[H]–TdR incorporation was measured. (E-M) 3 × 106 MACS-purified CD19+ B cells from Eµ-myc+L mice were transplanted into BL/6 recipients and mice were treated with 200 μg αPD-1 mAb (blue bars) or control IgG (yellow bars) i.p. every third day. Day 17 after treatment, splenocytes were isolated and (E) frequencies and (F) absolute numbers of CD4+ and CD8+ T cells were analyzed using flow cytometry. (G) MACS purified CD8+ T cells were activated with plate-bound αCD3 mAb (2 µg/ml) and 3[H]–TdR incorporation was measured. (H-K) MACS purified CD4+ and CD8+ T cells were stimulated with PMA/Ionomycin for 5 h in vitro. Then, IFN-γ and TNF-α production was measured by flow-cytometry. (H, I) Total numbers and (J, K) MFI index of IFN-γ− and TNF-α-producing T cells is shown. (L) Absolute CD19+ B cell numbers (n = 3–5 mice/group). (M) Kaplan-Meier curve (n = 5 mice/group). (N) 3 × 106 MACS-purified CD19+ B cells from Eµ-myc+L mice were transplanted into BL/6 or PD-1−/− recipients and survival was monitored. Kaplan-Meier curve (n = 5 mice/group). Expression values for J, K are displayed as MFI index ( = intensity of expression in cytokine positive/cytokine negative cells). Data are displayed as mean ± SEM. Statistics: (B-L) Student's t test; (M, N) log-rank test. *p < 0.05, **p < 0.01, ***p < 0.0001. See also Supplementary Figure S4.
![Figure 4. Blockade of PD-1 signaling partially restores T cell function. (A) PDL-1 expression was analyzed on CD19+, CD11b+ or CD11c+ cells on splenocytes from BL/6 or Eµ-myc+L mice by flow-cytometry. One representative histogram out of 3 is shown. (black: staining, gray: isotype; n = 3 mice/group) (B) MACS-purified CD8+ T cells from naive BL/6 mice (responders) were activated with plate-bound αCD3 mAb (2 µg/ml) in the presence of titrated numbers of MACS-purified CD19+ B cells from naïve BL/6 or Eµ-myc+L mice for 72 h in triplicate cultures. 3[H]–TdR was added to the culture for the last 18 h of incubation. 1 representative out of 12 experiments is shown. (C) CD8+ T cells were cultured with titrated amounts of MACS-purified CD19+ B cells from Eµ-myc+L in the absence or presence of a blocking αPD-1 mAb (10 μg/ml) or control IgG and proliferation was measured as described in (B). Naïve BL/6 CD8+ T cells were cultured in the presence or absence of a αPD-1 or isotype control mAb served as controls. (D) Titrated numbers of MACS purified, irradiated (1000 rad) CD11b+ and CD11c+ APCs were cultured with CD8+ T cell responders (as described in (B) in the presence or absence (hatched bars) of an αPD-1 mAb (10 μg/ml) or control IgG and 3[H]–TdR incorporation was measured. (E-M) 3 × 106 MACS-purified CD19+ B cells from Eµ-myc+L mice were transplanted into BL/6 recipients and mice were treated with 200 μg αPD-1 mAb (blue bars) or control IgG (yellow bars) i.p. every third day. Day 17 after treatment, splenocytes were isolated and (E) frequencies and (F) absolute numbers of CD4+ and CD8+ T cells were analyzed using flow cytometry. (G) MACS purified CD8+ T cells were activated with plate-bound αCD3 mAb (2 µg/ml) and 3[H]–TdR incorporation was measured. (H-K) MACS purified CD4+ and CD8+ T cells were stimulated with PMA/Ionomycin for 5 h in vitro. Then, IFN-γ and TNF-α production was measured by flow-cytometry. (H, I) Total numbers and (J, K) MFI index of IFN-γ− and TNF-α-producing T cells is shown. (L) Absolute CD19+ B cell numbers (n = 3–5 mice/group). (M) Kaplan-Meier curve (n = 5 mice/group). (N) 3 × 106 MACS-purified CD19+ B cells from Eµ-myc+L mice were transplanted into BL/6 or PD-1−/− recipients and survival was monitored. Kaplan-Meier curve (n = 5 mice/group). Expression values for J, K are displayed as MFI index ( = intensity of expression in cytokine positive/cytokine negative cells). Data are displayed as mean ± SEM. Statistics: (B-L) Student's t test; (M, N) log-rank test. *p < 0.05, **p < 0.01, ***p < 0.0001. See also Supplementary Figure S4.](/cms/asset/a1493c74-ecb6-4e01-8b3f-8bbbbe1ea9e7/koni_a_1365997_f0004_oc.gif)
Figure 5. Lymphoma B cells induce mouse and human CD8+ T cell dysfunction by secreting a soluble compound smaller than 1kDa. (A) MACS-purified CD8+ T cells from naive BL/6 mice (responders) were activated with plate-bound αCD3 mAb (2 µg/ml) in the presence of titrated numbers of MACS-purified CD19+ B cells from naïve BL/6 or Eµ-myc+L mice for 72 h in transwell tissue culture plates in triplicate cultures and 3[H]–TdR incorporation was determined after 72 h of culture. 1 representative out of 3 experiments is shown. (B) BL/6 CD8+ T cells were stimulated with αCD3 mAb as described in (A) in the presence and absence of titrated amounts of serum from Eµ-myc+L mice (n = 2 different sera) and 3[H]–TdR incorporation was determined after 72 h of culture. (C) Mouse and human naïve CD8+ T cells were stimulated with αCD3 mAb as described in (A) or OKT-3 (2 µg/ml) respectively, in the presence and absence of titrated amounts of B cell conditioned medium (BCM), derived from BL/6 or Eµ-myc+L B cell cultures. 3[H]–TdR incorporation was determined after 72 h of culture. One representative out of 3 experiments is shown. (D) CD8+ T cells were labeled with CFSE and CFSE dilution was measured after stimulation of T cells with αCD3 mAb in the presence and absence of indicated BCMs at a dilution of 1:3 for 72 h. 1 representative out of 2 experiments is shown. (E-G) CD8+ T cells were cultured as described in (A) in the presence and absence of titrated amounts of (E) untouched or heat-inactivated BCM and (F, G) size fractionated BCM from BL/6 or Eµ-myc+L B cell cultures and 3[H]–TdR incorporation was measured. 1 representative out of 6 experiments is shown. (H) Naïve BL/6 cells were cultured in the presence or absence of size fractionated BCM together with αCD3 mAb and viability was determined by Annexin-V staining after 24 h of culture. Apoptosis rate of CD8+ T cells in medium was 41% (data not shown). 1 representative out of 2 experiments is shown. (I) Naïve BL/6 cells were cultured in the presence or absence of size fractionated BCM and αCD3 mAb for 72 h. Then, IFN-γ and TNF-α production was measured by flow-cytometry after additional 5 h of in vitro stimulation with PMA/Ionomycin. In medium, TNF-α production of CD8+ T cells was 29%, IFN-γ production was 18% (data not shown). Data are displayed as mean ± SEM. Statistics: Student's t test.*p < 0.05, **p < 0.01, ***p < 0.0001. See also Supplementary Figure S5.
![Figure 5. Lymphoma B cells induce mouse and human CD8+ T cell dysfunction by secreting a soluble compound smaller than 1kDa. (A) MACS-purified CD8+ T cells from naive BL/6 mice (responders) were activated with plate-bound αCD3 mAb (2 µg/ml) in the presence of titrated numbers of MACS-purified CD19+ B cells from naïve BL/6 or Eµ-myc+L mice for 72 h in transwell tissue culture plates in triplicate cultures and 3[H]–TdR incorporation was determined after 72 h of culture. 1 representative out of 3 experiments is shown. (B) BL/6 CD8+ T cells were stimulated with αCD3 mAb as described in (A) in the presence and absence of titrated amounts of serum from Eµ-myc+L mice (n = 2 different sera) and 3[H]–TdR incorporation was determined after 72 h of culture. (C) Mouse and human naïve CD8+ T cells were stimulated with αCD3 mAb as described in (A) or OKT-3 (2 µg/ml) respectively, in the presence and absence of titrated amounts of B cell conditioned medium (BCM), derived from BL/6 or Eµ-myc+L B cell cultures. 3[H]–TdR incorporation was determined after 72 h of culture. One representative out of 3 experiments is shown. (D) CD8+ T cells were labeled with CFSE and CFSE dilution was measured after stimulation of T cells with αCD3 mAb in the presence and absence of indicated BCMs at a dilution of 1:3 for 72 h. 1 representative out of 2 experiments is shown. (E-G) CD8+ T cells were cultured as described in (A) in the presence and absence of titrated amounts of (E) untouched or heat-inactivated BCM and (F, G) size fractionated BCM from BL/6 or Eµ-myc+L B cell cultures and 3[H]–TdR incorporation was measured. 1 representative out of 6 experiments is shown. (H) Naïve BL/6 cells were cultured in the presence or absence of size fractionated BCM together with αCD3 mAb and viability was determined by Annexin-V staining after 24 h of culture. Apoptosis rate of CD8+ T cells in medium was 41% (data not shown). 1 representative out of 2 experiments is shown. (I) Naïve BL/6 cells were cultured in the presence or absence of size fractionated BCM and αCD3 mAb for 72 h. Then, IFN-γ and TNF-α production was measured by flow-cytometry after additional 5 h of in vitro stimulation with PMA/Ionomycin. In medium, TNF-α production of CD8+ T cells was 29%, IFN-γ production was 18% (data not shown). Data are displayed as mean ± SEM. Statistics: Student's t test.*p < 0.05, **p < 0.01, ***p < 0.0001. See also Supplementary Figure S5.](/cms/asset/a1498b62-336e-40d0-a6e5-d0e0d793a115/koni_a_1365997_f0005_oc.gif)
Figure 6. Lymphoma B cells produce and secrete purines that inhibit T cell function. (A) <1kDa fractions of BCM from BL/6 or Eµ-myc+L mice (n = 4 mice/group) were analyzed for metabolites using mass spectrometry. Results are indicated as S-Plot correlation of equal masses, detected in BL/6 ( = −1) or Eµ-myc+L ( = +1) mice. Masses with a very high (Eµ-myc+L = +1) or very low (BL/6 = −1) correlation reflect the highest significance. Masses selected for further screening are displayed in red and depicted in . (B) NMR analysis. (C) NMR signal after spike in of Hypoxanthine standard or Eµ-myc+L BCM (n = 4 mice/group). (D, E) Serum concentration of (D) ATP and (E) uric acid (n = 3 mice/group). (F) ATP concentrations in MACS-purified B cell lysates (n = 3 lysates/group). Concentrations of indicated purines in BCM (n = 3–5 BCM/group). Proliferation of purified, naïve CD8+ T cells from BL/6 mice was determined after addition of titrated amounts of different purines. (G) Titrated amounts of ATP, 5′ADP or AMP were added separately or combined (ATP + 5′ADP and ATP+5′ADP+AMP) to purified, naïve CD8+ T cells from BL/6 mice and 3[H]-TdR incorporation was determined. Data are presented as mean value of triplicate measurements and one representative experiment out of 2 independent experiments is shown. Data are displayed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.0001 (Student's t test); ((G) One-way ANOVA). See also Supplementary Figure S5-S7.
![Figure 6. Lymphoma B cells produce and secrete purines that inhibit T cell function. (A) <1kDa fractions of BCM from BL/6 or Eµ-myc+L mice (n = 4 mice/group) were analyzed for metabolites using mass spectrometry. Results are indicated as S-Plot correlation of equal masses, detected in BL/6 ( = −1) or Eµ-myc+L ( = +1) mice. Masses with a very high (Eµ-myc+L = +1) or very low (BL/6 = −1) correlation reflect the highest significance. Masses selected for further screening are displayed in red and depicted in Table 1. (B) NMR analysis. (C) NMR signal after spike in of Hypoxanthine standard or Eµ-myc+L BCM (n = 4 mice/group). (D, E) Serum concentration of (D) ATP and (E) uric acid (n = 3 mice/group). (F) ATP concentrations in MACS-purified B cell lysates (n = 3 lysates/group). Concentrations of indicated purines in BCM (n = 3–5 BCM/group). Proliferation of purified, naïve CD8+ T cells from BL/6 mice was determined after addition of titrated amounts of different purines. (G) Titrated amounts of ATP, 5′ADP or AMP were added separately or combined (ATP + 5′ADP and ATP+5′ADP+AMP) to purified, naïve CD8+ T cells from BL/6 mice and 3[H]-TdR incorporation was determined. Data are presented as mean value of triplicate measurements and one representative experiment out of 2 independent experiments is shown. Data are displayed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.0001 (Student's t test); ((G) One-way ANOVA). See also Supplementary Figure S5-S7.](/cms/asset/5206d434-2162-41c5-bfaa-7fb282fadf01/koni_a_1365997_f0006_oc.gif)
Table 1. Identification of lipids and members of the purine pathway in BCM from Eµ-myc+L mice. <1kDA fractions of BCM from Eµ-myc+L mice or BL/6 mice were analyzed for metabolites using Mass Spectrometry (as described in material and methods). Masses that appeared with highest intensity and significance in Eµ-myc+L BCM were further verified by fragmentation analysis. Identified masses contained bioactive lipids and members of the purine pathway, listed in , (n = 4 mice/group).
Figure 7. Metabolites of the purine degradation pathway are released by apoptotic lymphoma B cells and induce CD8+ T cell dysfunction. (A, B) MACS-purified B cells from Eµ-myc+L mice were cultured in the presence or absence of the caspase inhibitor QVD (50 µM) and BCM was harvested after indicated time points. (A) The concentrations of ATP, hypoxanthine, xanthine and thymidine in BCM from Eµ-myc+L mice were determined 1 h (ATP) and 24 h (hypoxanthine, xanthine and thymidine) after QVD or control (DMSO) treatment. (B) BCM was harvested 6 h after incubation and added to CD8+ T cell responders at a 1:1 ratio and 3[H]–TdR incorporation was measured. 1 representative out of 2 experiments is shown. (C) MACS-purified B cells from Eµ-myc+L mice were cultured in the presence or absence of the ATP degrading enzyme apyrase at different concentrations. BCMs were harvested 24 h after in vitro culture. BCMs were added to BL/6 CD8+ T cell responders at a 1:3 ratio and 3[H]–TdR incorporation was measured. 1 representative out of 3 experiments is shown. (D) CD4+ and CD8+ T cells from spleens of BL/6 or Eµ-myc+L mice were analyzed for the expression of ATP ectonucleotidases CD39 and CD73 using flow cytometry ex vivo. (n = 2; black: staining, gray: isotype, MFI: MFI staining – MFI isotype). One representative FACS plot out of 4 independent experiments is shown. (E, F) CD8+ T cells responders from naïve BL/6 or (E) CD39−/− (red bars) or (F) A2A−/− (green bars) mice were cultured in the presence or absence of Eµ-myc+L BCM at a 1:3 ratio and 3[H]–TdR incorporation was measured. Data are presented as mean value of triplicate measurements and one representative experiment out of 2 independent experiments is shown. (G) Spleen weights, (H) total splenocyte numbers and (I) T cell/B cell ratios of Eµ-myc x CD39−/−+L or Eµ-myc x CD39+/−+L. (J, K) MACS-purified (J) CD4+ and (K) CD8+ T cells were isolated from Eµ-myc x CD39−/−+L or Eµ-myc x CD39+/−+L and 3[H]–TdR incorporation was measured (n = 2 mice/group). (L) Kaplan-Meier survival curve of Eµ-myc x CD39+/−+L (n = 30) and Eµ-myc x CD39−/−+L (n = 4). Survival is depicted as Kaplan-Meier curve (n = 5 mice/group). Data are displayed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.0001 (Student's t test). See also Supplementary Figure S8 and S9.
![Figure 7. Metabolites of the purine degradation pathway are released by apoptotic lymphoma B cells and induce CD8+ T cell dysfunction. (A, B) MACS-purified B cells from Eµ-myc+L mice were cultured in the presence or absence of the caspase inhibitor QVD (50 µM) and BCM was harvested after indicated time points. (A) The concentrations of ATP, hypoxanthine, xanthine and thymidine in BCM from Eµ-myc+L mice were determined 1 h (ATP) and 24 h (hypoxanthine, xanthine and thymidine) after QVD or control (DMSO) treatment. (B) BCM was harvested 6 h after incubation and added to CD8+ T cell responders at a 1:1 ratio and 3[H]–TdR incorporation was measured. 1 representative out of 2 experiments is shown. (C) MACS-purified B cells from Eµ-myc+L mice were cultured in the presence or absence of the ATP degrading enzyme apyrase at different concentrations. BCMs were harvested 24 h after in vitro culture. BCMs were added to BL/6 CD8+ T cell responders at a 1:3 ratio and 3[H]–TdR incorporation was measured. 1 representative out of 3 experiments is shown. (D) CD4+ and CD8+ T cells from spleens of BL/6 or Eµ-myc+L mice were analyzed for the expression of ATP ectonucleotidases CD39 and CD73 using flow cytometry ex vivo. (n = 2; black: staining, gray: isotype, MFI: MFI staining – MFI isotype). One representative FACS plot out of 4 independent experiments is shown. (E, F) CD8+ T cells responders from naïve BL/6 or (E) CD39−/− (red bars) or (F) A2A−/− (green bars) mice were cultured in the presence or absence of Eµ-myc+L BCM at a 1:3 ratio and 3[H]–TdR incorporation was measured. Data are presented as mean value of triplicate measurements and one representative experiment out of 2 independent experiments is shown. (G) Spleen weights, (H) total splenocyte numbers and (I) T cell/B cell ratios of Eµ-myc x CD39−/−+L or Eµ-myc x CD39+/−+L. (J, K) MACS-purified (J) CD4+ and (K) CD8+ T cells were isolated from Eµ-myc x CD39−/−+L or Eµ-myc x CD39+/−+L and 3[H]–TdR incorporation was measured (n = 2 mice/group). (L) Kaplan-Meier survival curve of Eµ-myc x CD39+/−+L (n = 30) and Eµ-myc x CD39−/−+L (n = 4). Survival is depicted as Kaplan-Meier curve (n = 5 mice/group). Data are displayed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.0001 (Student's t test). See also Supplementary Figure S8 and S9.](/cms/asset/fabfda10-6bb0-4e17-b50b-69ec2cca17b6/koni_a_1365997_f0007_oc.gif)
