Figures & data
Figure 1. Pam2CSK4 sustains the survival of CD11b+Gr1+ MDSCs. (A) EG7 tumor-bearing mice were subcutaneously injected twice with PBS or 50 nmol Pam2CSK4 and OVA protein every 4 days. After 24 hours from last injection, the proportion of CD11b+Gr1+ cells in the spleen was analyzed by flow cytometry. Numbers adjacent to outlined areas indicate the percentage of relevant population. (B) CD11b+Gr1+ cells were isolated from EG7 tumor-bearing B6 WT, TLR2−/−, or IL-6−/− mice, and cultured in the presence of PBS or Pam2CSK4. After 24 h, cell viability was measured by WST-1 assay. (C) CD11b+Gr1+ cells treated with PBS (thin line histogram) or Pam2CSK4 (bold line histogram) for 24 hours were analyzed by flow cytometry after staining with PI. (D) Real-time PCR analysis of transcripts for Bcl2l1, Bcl3, Ccnd1, Ccnd2, ler3, and c-Myc in CD11b+Gr1+ cells isolated from tumor-bearing B6 WT or TLR2−/− mice after in vitro treatment with Pam2CSK4 or PBS for 4 h. (E) Flow cytometric analysis of Ly6G and Ly6C expression in CD11b+Gr1+ cells (left). TLR2 expression in G-MDSCs or M-MDSCs (right). (F) G-MDSCs and M-MDSCs were isolated from tumor-bearing mice and incubated with Pam2CSK4 or PBS for 24 h. Cell viability was measured by WST-1 assay. Data represent means ± standard deviation (SD) in graph. n = 3. **P < 0.005. *P < 0.05. All data shown are representative of more than 2 independent experiments.
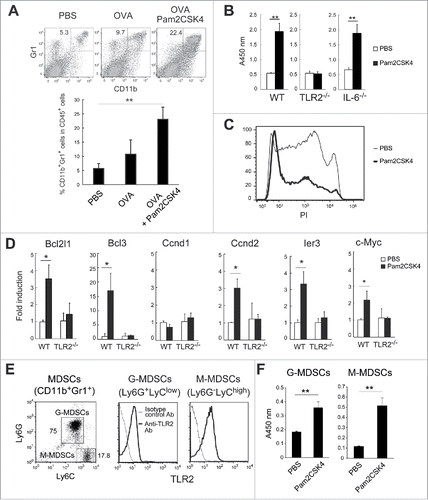
Figure 2. CD11b+Gr1+ cells differentiate into F4/80+/CD115+ macrophages in vivo and in vitro. (A) CFSE-labeled CD11b+Gr1+ cells (1 × 107) isolated from tumor-bearing mice were adoptively transferred into tumor-bearing mice. After 1 h, the mice were injected intravenously with PBS or 50 nmol Pam2CSK4. After 24 h, splenocytes were analyzed by flow cytometry. CFSE+ cells were gated and examined for CD11b and Gr1 expression. CD11b+Gr1− cells were further gated (bold line) and analyzed for F4/80 or CD115 expression. Numbers adjacent to outlined areas or above the brackets indicate the percentage of relevant population. (B) CD11b+Ly6G+Ly6Clow cells or CD11b+Ly6G−Ly6Chigh cells isolated from tumor-bearing mice were cultured in the presence or absence of Pam2CSK4. After 24 h, cells were analyzed for expression of Gr1, F4/80, and CD115. Numbers represent the percentage of gated cells. All data shown are representative of 2 independent experiments.
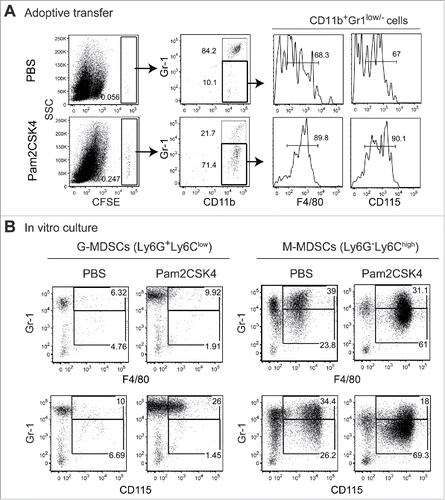
Figure 3. Pam2CSK4 enhances immunosuppressive activity of M-MDSCs but not G-MDSCs in vitro. (A and B) CD11b+Gr1+ cells isolated from EG7 tumor-bearing mice were cultured with CFSE-labeled CD8+ OT-I T cells (0.5 × 105), CD11c+ DCs (0.5 × 105), and SL8 peptide in the presence of 100 nM Pam2CSK4 or PBS. CD11b+Gr1+ cells were added to the cultures at the indicated ratios. After 3 days, CFSE fluorescence of CD8+ T cells was analyzed by flow cytometry to measure antigen-specific T cell proliferation. CD11b+Gr1+ cells were isolated from B6 WT mice (A and B) or TLR2−/− mice (B) implanted with EG7 tumors. Numbers near the brackets indicate the percentage of relevant population (thin line). Gray histograms indicate non-proliferated T cells in the absence of SL8 peptide. Each plot represents mean ± SD. n = 4. **P < 0.005. (right panel of A) (C) CD11b+Gr1+ cells isolated from tumor-bearing mice were pretreated with 100 nM Pam2CSK4 for 4 h, washed with the culture medium, and cultured with T cells and DCs as described in A. (D and E) G-MDSCs (D) or M-MDSCs (E) were cultured with CD8+ OT-I T cells (0.5 × 105) and CD11c+ DCs (0.5 × 105) as described in (A). (F) M-MDSCs (1 × 105) were cultured with CD8+ OT-I T cells (0.5 × 105) and CD11c+ DCs (0.5 × 105). Data represent means ± SD. n = 4. **P < 0.005. All data shown are representative of more than 2 independent experiments.
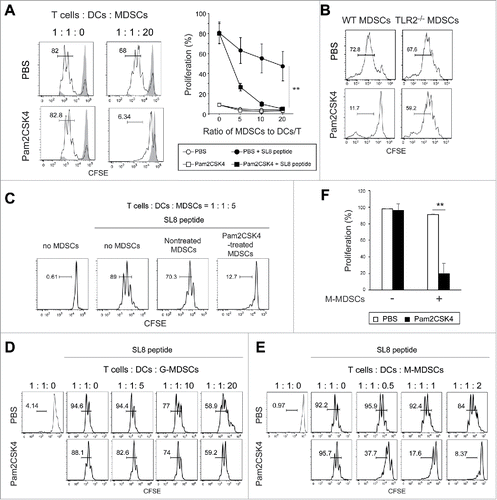
Figure 4. iNOS activity is essential for Pam2CSK4-enhanced suppressive activity. (A) L-NIL (50 nM) was added to cultures of M-MDSCs, CD8+ OT-I T cells, and CD11c+ DCs. T cell proliferation was analyzed by flow cytometry. Numbers above the brackets indicate the percentage of relevant population. (B) Nitrites in the conditioned media of the cocultures were measured by Griess reagent. Data represent means ± SD. n = 3. **P < 0.005. (C) M-MDSCs (1 × 105) were cultured with CD8+ OT-I T cells (0.5 × 105) in the presence or absence of SL8 peptide or Pam2CSK4. iNOS expression in F4/80+ and F4/80− cells was analyzed by flow cytometry. MT: M-MDSCs and T cells; MTS: M-MDSCs, T cells, and SL8 peptide; MTSP: M-MDSCs, T cells, SL8 peptide, and Pam2CSK4. (D) iNOS expression in F4/80+ cells and CD11c+ DCs in the cocultures was analyzed by flow cytometry. All data shown are representative of more than 2 independent experiments.
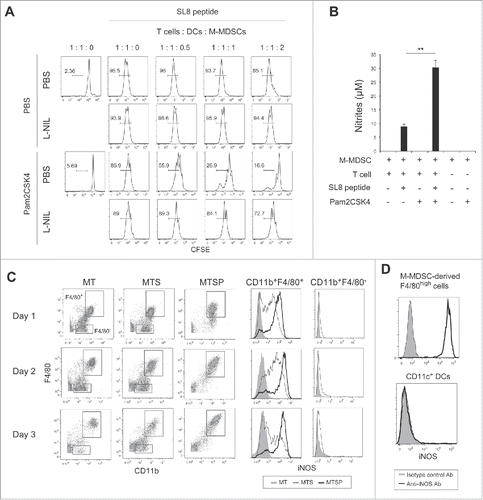
Figure 5. CD8+ T cell-produced IFN-γ induces iNOS expression in M-MDSC-derived macrophages. (A) The concentrations of IFN-γ (left panel) or IL-12p40 (right panel) in the conditioned media of CD8+ T cells cultured with M-MDSCs (ratio 1:2) were determined by ELISA. Data are shown as means ± SD. n = 3. *P < 0.05. (B) iNOS expression in F4/80+ cells after coculture of M-MDSCs with CD8+ OT-I T cells for 24 h in the presence of Pam2CSK4, anti-IFN-γ antibody, and/or isotype control antibody (isotype Ctrl.). Numbers above the brackets indicate the percentage of relevant population. (C) iNOS expression in F4/80+ cells derived from M-MDSCs cultured for 24 h in the presence of recombinant IFN-γ (200 U/mL) and Pam2CSK4. (D) IFNGR1 expression was determined in F4/80+ or F4/80− cells after 24-h culture in the presence of Pam2CSK4. Cells were stained with anti-IFNGR1 Ab (black line histogram) or isotype control Ab (gray-filled histogram). (E) iNOS expression in G-MDSCs after 24-h culture in the presence of IFN-γ (200 or 600 U/mL) and Pam2CSK4. (F) IFNGR1 expression in G-MDSCs. Cells were stained with anti-IFNGR1 Ab (black line histogram) or isotype control Ab (gray-filled histogram). All data shown are representative of more than 2 independent experiments.
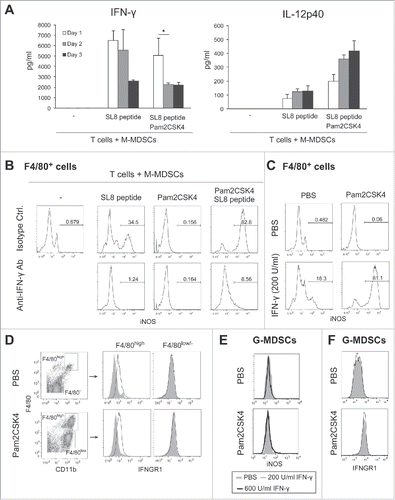
Figure 6. Blocking of iNOS activity augments the therapeutic potential of Pam2CSK4 in tumor-bearing mice. (A) B6 WT or TLR2−/− mice bearing EG7 tumors were treated with Pam2CSK4 and OVA protein. After 24 h, tumors were excised and iNOS expression in CD11b+F4/80+ cells was analyzed by flow cytometry. Numbers adjacent to outlined areas indicate the percentage of relevant population. (B) EG7 tumor-bearing mice were pretreated with anti-CD8β antibody for 24 h. Pam2CSK4 (Pam2) and OVA protein were injected into the mice. After 24 h, tumor lysate was prepared and IFN-γ concentration was measured by ELISA. IFN-γ levels were represented as IFN-γ (ng) /tumor (g). (C, D and E) B6 WT mice bearing EG7 (C) or LLC-OVA (D and E) tumors were treated with Pam2CSK4 and OVA protein as indicated by arrows. Mice were treated daily with L-NAME (2 mg) (C and D), L-NIL (0.5 mg) (E), or PBS. Data are shown as means ± SD. n = 4-5. *p < 0.05. All data shown are representative of more than 2 independent experiments.
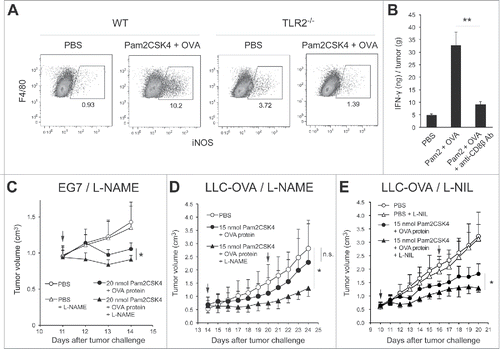
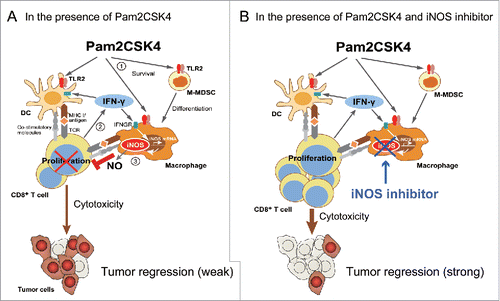
Figure 7. Putative mechanism of Pam2CSK4-enhanced immunosuppression by M-MDSCs. (A) M-MDSCs differentiate into macrophages. Pam2CSK4 increases their survival and differentiation through TLR2 signaling (Step 1). M-MDSCs and/or macrophages present peptide to CD8+ T cells, and transiently activate them to produce IFN-γ (Step 2). IFN-γ induces iNOS expression in macrophages differentiated from M-MDSCs. iNOS-generated NO inhibits T cell proliferation induced by DCs (Step 3). In contrast to M-MDSCs, DCs undergo maturation and activation in the presence of Pam2CSK4 and IFN-γ, leading to T cell proliferation. (B) By using iNOS inhibitor, NO-mediated T cell suppression by Pam2CSK4-activated M-MDSCs/macrophages is abrogated, thereby DCs can strongly induces the activation/proliferation of anti-tumor CD8+ T cells, leading to tumor regression.