Figures & data
Figure 1. Schematic representation and three-dimensional model of the anti-EGFR x anti-CD3 ATTACK. (A) Schematic diagrams showing the genetic (left) and domain structure (right) of the ATTACK molecule, bearing a signal peptide from the oncostatin M (white box), three anti-EGFR EgA1 VHH genes (red boxes) and three collagen-derived trimerization (TIE) domains flanked peptide linkers (pale green boxes), the anti-CD3 OKT3 scFv gene (blue boxes), and epitope tags (yellow box). Arrows indicate the direction of transcription. (B) Three-dimensional model of the ATTACK molecule.
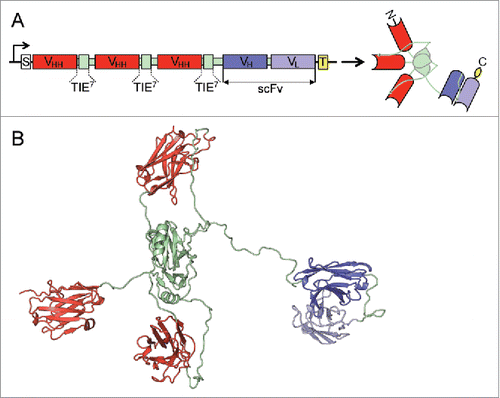
Figure 2. Structural characterization of purified EgA1 ATTACK. Reducing SDS-PAGE (A) and SEC-MALS (B) with the indicated molecular masses measured at the center of the chromatography peaks. The black line corresponds to the UV absorbance (left axis) and the red line to the measured molar mass (right axis). Circular dichroism spectrum (C) and tertiary structure analysis by thermal denaturation measured by the change in ellipticity at 210 nm (D).
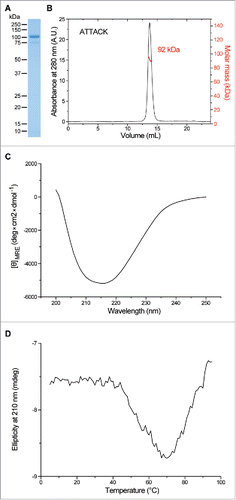
Figure 3. Functional characterization of purified EgA1 ATTACK. (A) Reducing SDS-PAGE of the purified EgA1 VHH, EgA1 multichain N-trimerbody (EgA1N), EgA1 ATTACK and EGFR-Fc. (B and C) Biolayer interferometry (BLI)-derived sensorgrams (in black) for the interaction between immobilized EGFR-Fc and cetuximab- (B) or EgA1-based (C) antibodies. Fitting curves for cetuximab-Fab and EgA1-VHH are included in red. (D) The binding to EGFR on the cell surface of HeLa cells by cetuximab- and EgA1-based antibodies at 0.1, 0.32, 1, and 10 nM, measured by FACS and normalized to the binding at 10 nM. (E) The binding to CD3 on the cell surface of Jurkat cells by OKT3- and EgA1-based antibodies at 0.1, 1, 3.2, and 10 nM, measured by FACS and normalized to the binding at 10 nM.
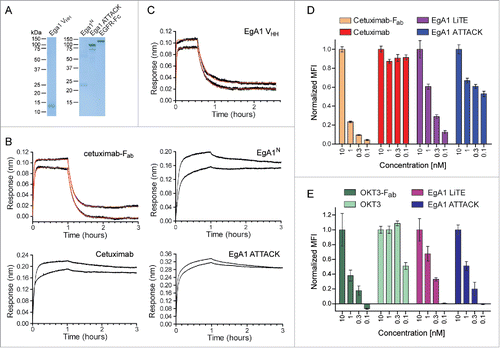
Figure 4. Immunological synapse formation is triggered by EgA1 ATTACK. (A) IS formation assessed by confocal microscopy. Green (CD3ζ) and red (actin) channels of a confocal section are shown with a pseudo-color displaying the range of intensity values. Calibration bar for pseudo-color applied in all images is shown in the first panel. Images of merged channels are shown, where CD3ζ is presented in green, actin in red and 3T3-EGFR cells in blue. As a control, Jurkat T cells were incubated together with 3T3-EGFR cells in the absence of antibodies. Scale bar 5 μm. (B) Quantification of CD3ζ (left graph) and actin (right graph) polarization towards the IS in Jurkat T cells. Dots in graphs represent individual cells obtained from several experiments. Results are expressed as mean ± S.D. (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns, not significant). The experiments were performed three times and results of one representative experiment are shown.
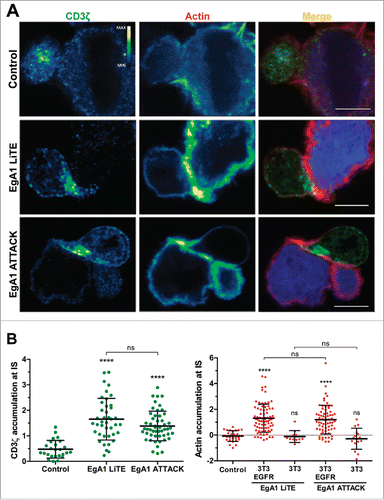
Figure 5. Activation of downstream TCR signaling by EgA1 ATTACK. (A) Polarised activation of PLCγ assessed by confocal microscopy. Green (pPLCγ) and red (actin) channels of a confocal section are shown with a pseudo-color displaying the range of intensity values. Calibration bar for pseudo-color applied in all images is shown in the first panel. Images of merged channels are shown, where pPLCγ is presented in green, actin in red and 3T3-EGFR cells in blue. Scale bar 5 μm. (B) Quantification of pPLCγ activation at the immunological synapse. As a control, Jurkat T cells were incubated together with 3T3-EGFR cells in absence of antibodies. Dots represent individual cells of one experiment. (C) Western blot analysis for ERK1/2 and PLCγ activation. Densitometric analyses were performed, and the ratio of the mean fluorescence intensity (MFI) of phosphoproteins and total proteins is shown for different concentrations of EgA1 LiTE or EgA1 ATTACK. Results are expressed as mean ± S.D (n = 3). (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Not significant differences are not displayed. The experiments were performed three times and results of one representative experiment are shown.
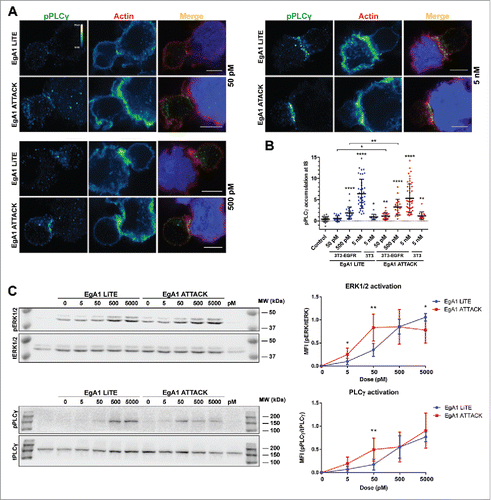
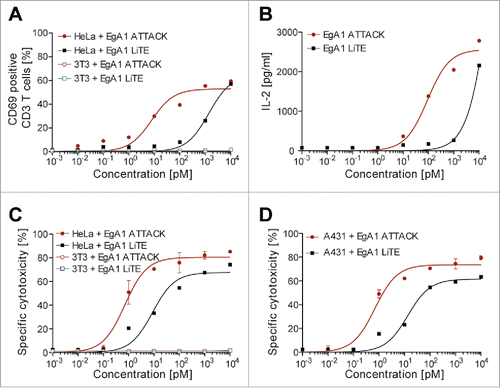
Figure 6. Induction of T cell activation and cytotoxicity by EgA1 ATTACK. EGFR-positive HeLa cells or EGFR-negative 3T3 cells were co-cultured in 96-well plates with PBMCs in the effector:target (E:T) ratio of 5:1, and purified EgA1 LiTE or EgA1 ATTACK. After 24 hours, the surface expression of T-cell activation surface marker CD69 was determined by FACS analysis (A) and IL-2 production was determined by ELISA (B). Specific lysis of HeLaLuc cells and 3T3Luc cells (C) or A431Luc cells (B) incubated with PBMC (5:1) in the presence of purified antibodies and after 48 h. Percent viability was calculated relative to the luminescence from an equal number of input control cells and used to calculate percent specific lysis. Results are expressed as a mean ± SD (n = 3) from 1 of at least 3 separate experiments. The experiments were performed three times and results of one representative experiment are shown.