Figures & data
Figure 1. 8Gyx2 and 2Gyx10 IR alone results in similar degrees of primary tumor control. A, 5 × 106 MOC1 or 1 × 105 MC38-CEA cells were injected subq into the legs of WT B6 mice (n = 10 mice/group), allowed to engraft, and treated with IR starting day 10 (black arrow). Primary tumor growth was measured 2–3/week. Shown are results from one of three independent experiments, each with similar results. B, schema for acquisition of primary tumor and spleens for immune correlative analysis.
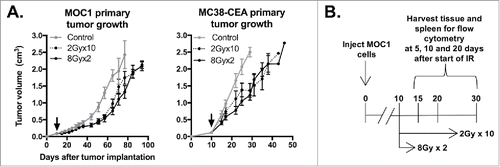
Figure 2. 8Gyx2 and 2Gyx10 IR variably alters effector immune infiltration and activation within the TME. Primary tumor tissues (n = 5/time point) were harvested 5, 10 and 20 days after the start of either 8Gyx2 IR (black lines), 2Gyx10 IR (grey lines) or no IR (dashed lines) and prepared single cell suspensions were analyzed via flow cytometric analysis. A, Effector immune cell infiltration into MOC1 primary tumors. B, Effector immune cell infiltration into MC38-CEA primary tumors. Quantification of tumor infiltration of immune cells presented as absolute number of cells per 1 × 104 live cells analyzed. Quantification of expression of specific markers presented as MFI. Cell subset definitions included CD8 TIL (CD3+CD8+), CD4 TIL (CD3+CD4+FoxP3−), NK (CD3−NK1.1+), DC (CD11 c+, CD11b+/−, PDCA+/−) and macrophages (CD11b+F4/80+CD11 c−). All cells were CD45.2+ and 7AAD−. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (t test) compared to control tumors for each time point.
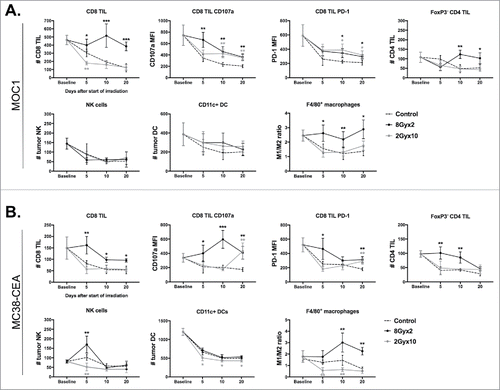
Figure 3. 8Gyx2 and 2Gyx10 IR variably alters tumor and peripheral accumulation of immunosuppressive cell subsets. Primary tumor and splenic tissues (n = 5/time point) were harvested and analyzed as in . A, Immunosuppressive immune cell accumulation in MOC1 primary tumors and spleen. B, Immunosuppressive immune cell accumulation in MC38-CEA primary tumors and spleen. Quantification of tumor accumulation of cells presented as absolute number of cells per 1 × 104 live cells analyzed. Quantification of splenic accumulation of cells presented as percentage of live, CD45.2+ cells analyzed. Cell subset definitions included gMDSC (CD11b+F4/80−Ly6GhiLy6Cint), mMDSC (CD11b+ F4/80−Ly6GlowLy6Chi) and Tregs (CD3+CD4+FoxP3+CD25hi). *, p < 0.05; **, p < 0.01; ***, p < 0.001 (t test) compared to control tumors for each time point.
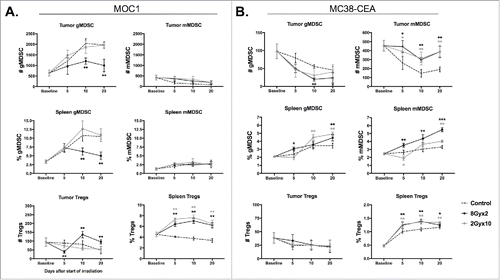
Figure 4. 8Gyx2 and 2Gyx10 IR variably alters IFN levels and expression of IFN-responsive MHC class I and PD-L1 within the TME. A, MOC1 (top panels) or MC38-CEA (bottom panels) primary tumor tissues (n = 5/time point) were harvested as in and analyzed via qRT-PCR for expression of type I IFN (IFNα/β) or effector (IFNγ/TNFα) cytokines. For all PCR data, B refers to baseline (day 10, before the start of IR) tumors. C (control), 2 × 10 Gy and 8Gyx2 tumors were collected and analyzed 10 days after the start of IR (tumor day 20). B, flow cytometric analysis of expression (MFI) of MHC class I (H2-Kb/Db) or PD-L1 on CD45.2−CD31−PDGFR− tumor cells within the TME. C, qRT-PCR analysis for expression of myeloid chemokines (VEGF/CXCL1). *, p < 0.05; **, p < 0.01; ***, p < 0.001 (t test) compared to control tumors for each time point.
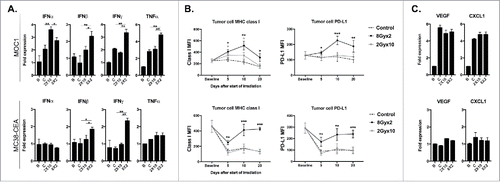
Figure 5. 8Gyx2 IR enhances while 2Gyx10 IR suppresses draining lymph node tumor-specific T-lymphocyte responses. T-lymphocytes were sorted from tumor draining lymph nodes 5, 10 and 20 days after the start of either 8Gyx2 IR, 2Gyx10 IR or no IR (n = 5/time point) and analyzed for tumor-specific immune responses. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (t test).
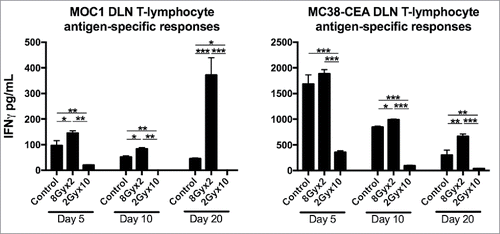
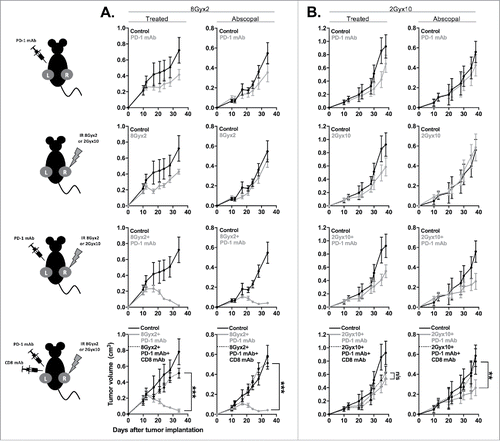
Figure 6. 8Gyx2 plus PD-1 mAb but not 2Gyx10 IR plus PD-1 mAb controls primary tumor growth and enhances survival. MOC1 (A) or MC38-CEA (B) tumors (n = 10 mice/group) were engrafted and treated with either 8Gyx2 or 2Gyx10 IR with or without PD-1 mAb (200 μg/injection, twice weekly × 3 weeks). IR and PD-1 mAb treatments were started concurrently on day 10. Individual tumor growth curves (top panels) and survival (bottom panels) are shown. Results shown are from one of two independent experiments with similar results. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (log-rank test).
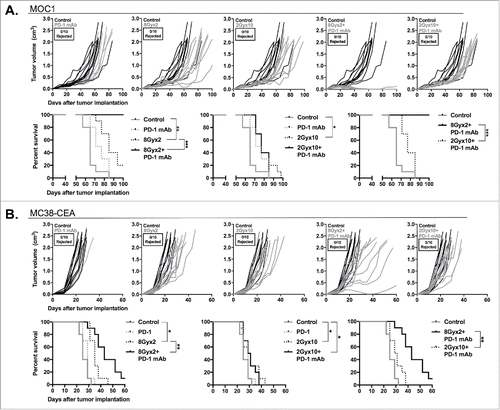
Figure 7. 8Gyx2 IR plus PD-1 mAb results in CD8+ cell-dependent control of primary and abscopal tumors. MOC1 tumors were simultaneously engrafted in the left leg (primary tumor) and right flank (abscopal tumor) of WT B6 mice (n = 5 mice/group). Mice were treated with 8Gyx2 (A) or 2Gyx10 IR (B) to the left leg tumor only, PD-1 mAb (200 μg/injection, twice weekly × 3 weeks), or the combination, and primary and abscopal tumor growth was measured. Results shown are from one of two independent experiments with similar results. In separate experiments, mice bearing primary and abscopal tumors were treated with combination IR (8Gyx2 or 2Gyx10) plus PD-1 mAb with or without CD8+ cellular depletion (clone YTS169.4, 200 μg/injection twice weekly for 2 weeks beginning the day before the start of treatment) and primary and abscopal tumor growth was measured (bottom panels). **, p < 0.01; ***, p < 0.001 (t test of day 35 tumor measurements).