Figures & data
Figure 1. Effects of CXCL11 transduction into Human CAR T cells. A) CXCL11 secretion: Human CAR T cells were transduced with the CXCL11 gene and placed into culture for 24 hours. As measured by ELISA assay, the CXCL11 transduced T cells secreted significantly (p < 0.001) more CXCL11. B) Chemotaxis: Activated human T cells were placed in the top of a Boyden chamber. In the lower well was placed cell media (R10) as a negative control, 10 ng/ml of recombinant human CXCL11 protein as a positive control, or conditioned media from the CAR/CXCL11 cells. Both recombinant CXCL11 and the CXCL11-conditioned media significantly (p<0.001) enhanced migration of human T cells compared to media control. C) CAR T cell Killing: Equal numbers of CAR or CAR/CXCL11 T cells were added to mesothelioma cells not expressing mesothelin (EMParental) or to mesothelioma cells expressing human mesothelin (EMMeso) at various T cell to tumor ratios. After overnight incubation, their ability to kill the target cells was determined.
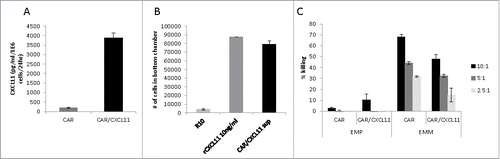
Figure 2. In vivo studies using CAR T cells. A) Tumor-bearing NSG mice were injected intravenously once with 10 million activated T cells that were either non-transduced (NTD), mesothelin-targeted CAR T cells (CAR-GFP), or CAR-CXCL11 T cells. Tumor size was followed over time. Data shown are means ± SEM, n = 5 mice per group. CAR-GFP T cells were significantly smaller than the mice receiving the NTD T cells (* = p < 0.05), with no effect elicited by he CAR-CXCL11 cells. B) On Day 22, tumors were harvested, homogenized and the amount of CXCL11 determined using an ELISA assay. C) In a second experiment, Group 1 animals received one dose of 10 million NTD T cells on Day 0 (arrow) and one dose on Day 11 (arrow). Group 2 mice received one dose of 10 million Meso-CAR T cells on Day 0 and one dose of Meso-CAR T cells on Day 11. Group 3 mice received 10 million CAR-CXCL11 CAR T cells on Day 0 and one dose of 10 million CAR T cells on Day 11. The growth of the tumors was followed until Day 24. Data shown are means ± SEM, n = 7 mice per group. The tumors in the Group 2 mice receiving of two doses of CAR T cells were significantly smaller than the NTD Group 1 mice (p < 0.001). The tumors in the Group 3 mice were significantly smaller than the NTD tumors (p < 0.01), however, the Group 3 tumors were significantly larger than the Group 2 tumors (p < 0.05). D). Tumors were harvested at the end of the study, digested, and the % of live human CD3 T cells measured by flow cytometry. E) Average percent of human CD3 T cells within the CAR vs CAR/CXCL11 groups is plotted. There were significantly more T cells in the CAR group (p < 0.001).
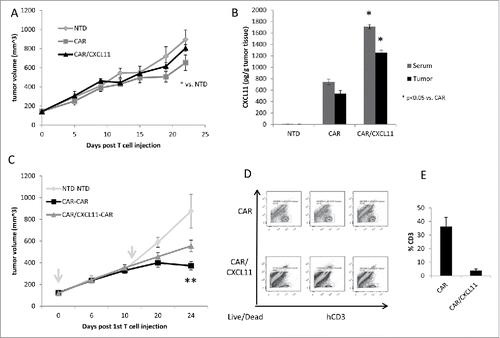
Figure 3. VV.CXCL11 administration into tumor-bearing mice led to robust CXCL11 generation, and tumor control. Wild type C57 Bl/6 mice were subcutaneously inoculated with TC1 murine lung cancer cells on the right flanks, and when tumors were established (approximately 200 mm3), they were treated with 108 pfu of VV.luc and VV.CXCL11, administered intravenously. A) Tumors were then monitored for the following 10–14 days. Data shown are means ± SEM, n = 5 mice per group. Tumors treated with both VV's were significantly smaller than control tumors (p < 0.05) but not different from one another. B) At Day 25, mice were euthanized, and tumors were excised for ex vivo analysis. Flow cytometry indicated a significant (** = p < 0.01) influx of CD8 tumor-infiltrating lymphocytes (TILs) in the VV.CXCL11-treated mice. C) Tumor tissues were processed and analyzed for CXCL11 production via ELISA. Values are reported in nanogram CXCL11 detected per gram of tumor tissue. CXCL11 levels were significantly (** = p < 0.01) higher in the VV.CXCL11 treated mice. D) The presence of other cytokines were also evaluated by ELISA to assess the specificity of CXCL11 production, and others that may be induced in this model. Values are reported in picogram of cytokines detected per gram of tumor tissue. There were no significant differences.
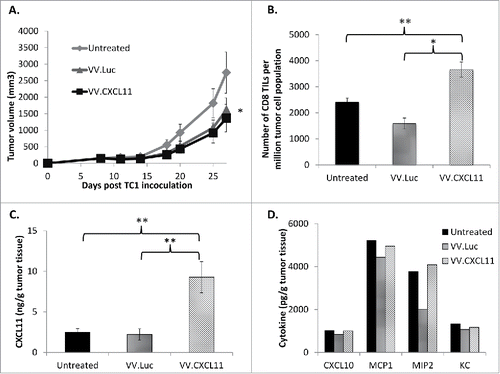
Figure 4. VV.CXCL11 significantly augmented an Ad.E7 cancer vaccine and increased T cell infiltration. A) TC1-bearing wild type mice were treated with Ad.E7 vaccine or vaccinia viruses – mice were vaccinated against E7 at Day 10 and again at Day 15, while vaccinia vectors were administered at Day 12. Tumor size was measured. Data shown are means ± SEM, n = 5 mice per group. A) Ad.E7 alone, VV.luc, VV.CXCL11, and Ad.E7 plus VV.luc all reduced the size of the TC1 tumors, but these changes did not reach statistical significance. In contrast, Ad.E7 plus VV.CXCL11 was the only group significantly smaller (p < 0.05) than control tumors. B) Survival curves from the same study, show that only the Ad.E7 plus VV.CXCL11 had prolonged survival (40%) (p < 0.05 using log-rank test). C) Tumors were harvested on Day 23. Ex vivo TIL analysis of tumors from each group revealed increased influxes of CD3 TILs in the Ad.E7 and Ad.E7 + VV.CXCL11 combination group. Data is expressed as means ± SEM, n = 5 mice per group (* = p < 0.05). D) The frequency of HPV-E7 positive CD8 TILs was determined. The percentage was significantly (p < 0.05) increased in the Ad.E7 + VV.CXCL11 combination group.
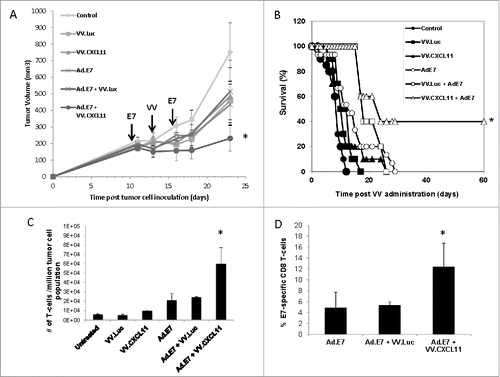
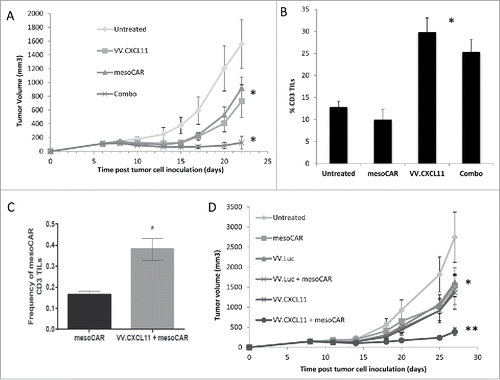
Figure 5. VV.CXCL11 significantly augmented mesoCAR immunotherapy. A) Mice bearing TC1-meso tumors were left untreated, injected iv with 108 pfu VV.CXCL11 on Day 5, injected iv with 107 mesothelin CAR-expressing T cells (iv) on day 8, or given both the iv VV.CXCL11 on Day 5 and the iv Meso-CAR T cells on Day 8. Tumor size was followed. Data is expressed as means ± SEM, n = 5 mice per group (* = p < 0.05). Both single treatments significantly (* = p < 0.05) reduced tumor size compared to control mice, however the combination was significantly (* = p < 0.05) more effective than either treatment alone. B) Tumors were digested and analyzed by flow cytometry on Day 22. The % of digested tumor cells that were CD3+ T cells was significantly higher in both groups receiving VV.CXCL11 (* = p < 0.05)). C) The % of adoptively transferred meso-CAR T cells present in the VV.CXCL11 treated tumors was more than double than that seen in the tumors treated with mesoCAR T cells alone (* = p < 0.05). D) In a separate experiment, groups received iv VV.luc alone and iv VV.luc plus iv Meso-CAR T cells in addition to the groups describe above in (A). Data is expressed as means ± SEM, n = 5 mice per group (* = p < 0.05). The combination of meso-CAR T cells plus VV.CXCL11 was again significantly better (p < 0.05) than all other treatments, however, administration of VV.luc did not augment the efficacy of Meso-CAR T cells. One way ANOVA with the appropriate post hoc testing, with * (P ≤ 0.05), ** (P ≤ 0.01). All experiments were replicated at least twice in an independent manner.