Figures & data
Figure 1. Expression of IL-25R and IL-25 in primary tumor cells from patients with FL, DLBCL or BL. (A) MNCs from B-NHL lymph nodes were double stained with anti-Ig κ or anti λ mAbs in combination with anti IL-17RA or anti IL-17RB mAbs, and analyzed by flow cytometry gating on the cell fraction expressing monoclonal κ or λ chains. Results for 10 FL, 6 DLCBL and 3 BL cases are shown in histograms, as mean % positive cells +SD. (B) Immunohistochemical analysis of IL-17RB in representative lymph nodes from patients with FL (X200 left panel and X400 right panel). IL-17RB is expressed in the neoplastic GC of FL. (C) Immunohistochemical analysis of IL-25 in representative lymphnodes from patients with FL. IL-25 expression is diffusely distributed among FL cells and associated microenvironment components (X200 left panel and X400 right panel). (D) Cell suspensions from B-NHL lymph nodes were surface stained with anti-Ig κ and anti λ mAbs, permeabilized, stained intracellularly with anti-IL-25 mAb and analyzed by flow cytometry gating on the cell fraction expressing monoclonal κ or λ chains. Results are means % positive cells from 5 FL, 3 DLCBL and 2 BL cases pooled together +SD.
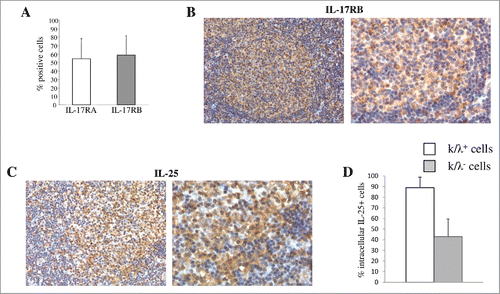
Figure 2. IL-25 signaling and function in B-NHL cells. (A) Flow cytometric analysis of pNFkBp65, pSTAT1 and pJNK in purified lymphoma cells (3 FL and3 DLBCL) treated for 0, 5 or 30 min with hrIL-25 (50 ng/ml). Results are shown as median MRFI, maximum, minimum and first and third quartile. (p = 0.0089 for pNF-kBp65, p = 0.0071 for pSTAT-1 at five minutes of treatment and p = 0.0024 for pJNK at 30 minutes of treatment). (B-C) Primary neoplastic cells from 5 FL and 3 DLBCL patients were cultured without (Med) or with rhIL-25 or hrCD40L and tested for proliferation by 3H-TdR incorporation (B) or by intracellular staining for PCNA (C). Results are expressed in box plot, as median cpm (B) or % positive cells (C), maximum, minimum, first and third quartile. (D) CAM assay. Gelatin sponges were adsorbed with: vehicle alone-PBS (left panel), hrIL-25 (middle panel) or VEGF-A (right panel). Original magnification: x 50.
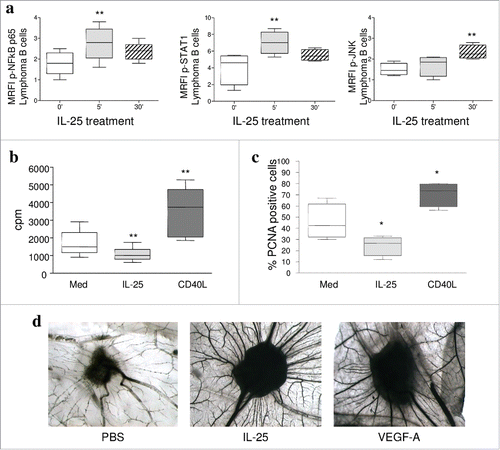
Figure 3. Role of IL-25 on in vivo B-NHL growth. (A) Volume of tumors grown s.c. in mice treated with PBS or IL-25 (1µg) 20 days after SU-DHL-4 cell injection. Results are expressed in box plot, as median tumor volume mm3, maximum, minimum and first and third quartile (**p = 0.002). (B) Tumors developed after s.c. injection of SU-DHL-4 cells in PBS treated SCID/NOD mice consisted of a mixture of small and large lymphoid cells with centrocyte and centroblast morphology (a). These tumors displayed a distinct vascularization (b), a strong proliferative activity (c) and some apoptotic events (d). The histologic features of SU-DHL-4 tumors appeared heavily compromised by rhIL-25 treatment, since these tumors were characterized by wide areas of ischemic necrosis (N) (e), defective vascularization (f), and decreased proliferation (g), whereas apoptotic events (h) were comparable to those observed in control tumors (d) (Magnification: X400). (C) Flow cytometric analysis of cell proliferation in cell suspensions from explanted SU-DHL-4 cell tumors, as assessed by Ki67 staining. Results for 6 PBS and 6 IL-25-treated mice are expressed as median % positive cells, maximum, minimum and first and third quartile (**p = 0.0049).
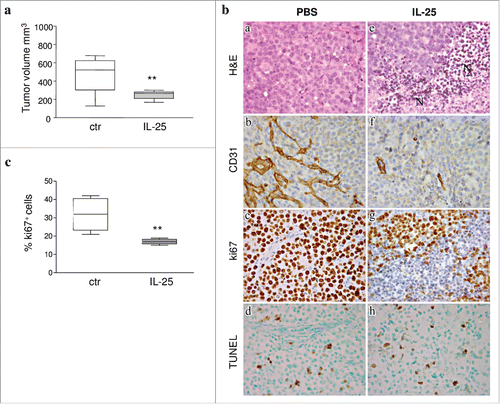
Table 1. Immunohistochemical assessment of microvessels, apoptotic cells and pro-angiogenic molecules in tumors developed after SU-DHL-4 cell injection in PBS or hrIL-25 treated mice.
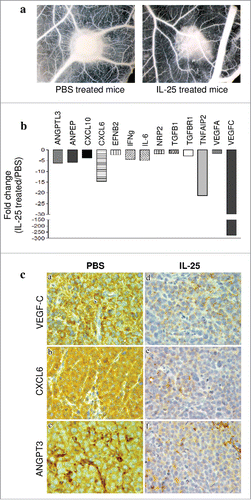
Figure 4. Role of IL-25 on in vivo tumor angiogenesis. (A) CAM assay performed ex vivo with tumor masses explanted from PBS (left panel) or hrIL-25 (right panel) treated mice. (B) Gene expression profiling of human angiogenesis related genes in SU-DHL-4 tumors explanted from SCID/NOD mice as assessed by PCR Array. Results represent fold differences in individual mRNA expression between PBS or hrIL-25 treated mice. Pooled results from 4 different experiments are shown. (C) Immunohistochemical analysis of the expression of selected pro-angiogenic molecules in SU-DHL-4 tumors from PBS or hrIL-25 treated mice. In comparison with tumors developed in PBS treated mice (a-c), the small tumor masses from hrIL-25 treated mice showed very low expression of VEGF-C (d), CXCL6 (e) and ANGPT3 (f).