Figures & data
Figure 1. QBKPN was efficacious in models of metastatic-like lung cancers. (A) LLC left lung surface tumor nodule counts and (B) tumor visualization using Bouin's fixative, in mice 14 days after lung tumor challenge via tail vein injection (day 0). QBKPN or placebo was administered subcutaneously every other day starting 10 days before tumor challenge, until the end of the experiment. (C) Survival following LLC challenge in mice administered with placebo or QBKPN from day −10 until euthanasia. (D) B16F10 lung surface tumor nodule counts, and (E), the melanoma specific Tyrosinase (Tyr) gene expression in the post-caval lung lobe, on day 17 post-tumor challenge (tail vein), in mice administered with placebo or QBKPN from day −10 until day 17. (F) Day 20 B16F10 lung surface tumor nodule counts of therapeutically treated mice (every-other-day placebo or QBKPN treatment initiated 1 day post-tumor challenge). (G) Day 14 B16F10 lung surface tumor nodule counts of therapeutically treated mice with treatment starting on day 5 post-tumor implantation. For tumor nodule counts and Tyr expression, mean +/− SD shown. N = 4–5 mice per group. *P < 0.05; **P < 0.01; ***P < 0.001 by Student's t-test. For Kaplan Myer survival plots, n = 10 mice per group, ** P < 0.01 by Log-rank test.
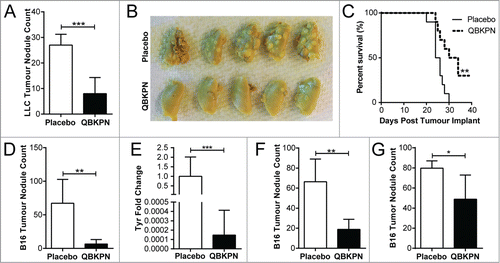
Figure 2. QBKPN anti-tumor efficacy required host exposure to Klebsiella. (A) LLC lung surface tumor nodule counts at day 18 in animals administered QBKPN or left untreated from day −10 to day 16, obtained from facilities testing positive for Klebsiella species or Klebsiella-excluded facilities. Klebsiella pre-infection of certain groups occurred 21 days before the beginning of the experiment (day −31). (B) Day 14 B16F10 lung surface tumor nodule count in mice pre-infected with Klebsiella >6 months prior to tumor challenge and administration of QBKPN (day −10 to day 12). (C) Day 18 LLC lung surface tumor nodule counts in mice pre-infected with Streptococcus pneumoniae or Pseudomonas aeruginosa (21 days before treatment, as described for Klebsiella) and administered a Streptococcus pneumoniae-derived immunotherapy (QBSPN) or a Pseudomonas-derived immunotherapy (QBPAE) from day −10 to day 16. (D) Day 18 LLC lung surface tumor nodule counts in mice with different Klebsiella exposures or Klebsiella pre-infections, administered Streptococcus pneumoniae QBSPN, or left untreated, from day −10 to day 16. SP = QBSPN; PA = QBPAE. Mean +/− SD shown, n = 4–8 mice per group. *, P < 0.05; **, P < 0.01; ****, P < 0.0001 by 1-way ANOVA followed by Tukey's multiple comparison test (A, C and D) or Student's t-test (B).
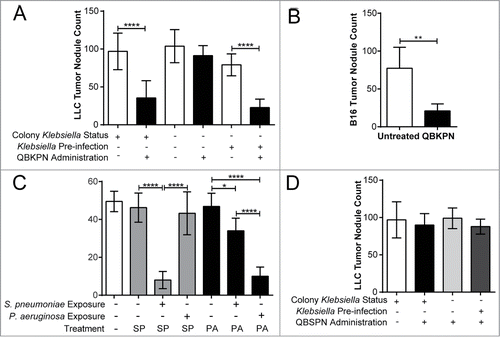
Figure 3. Adaptive immunity was dispensable for QBKPN anti-tumor efficacy. (A) B16F10 left lung surface tumor nodule counts of mice with or without CD25+ cell depletion, after administration of placebo or QBKPN from day −10 until euthanasia on day 18. (B) LLC left lung surface tumor nodule counts of mice with or without CD4+ cell depletion, after administration of placebo or QBKPN from day −10 until euthanasia on day 16. (C) B16F10 lung surface tumor nodule counts in Rag2 knockout mice administered with QBKPN or left untreated from day −10 until euthanasia on Day 14. Mean +/− SD shown, n = 4–5 mice per group. *, P < 0.05; **, P < 0.01 by Student's t-test.
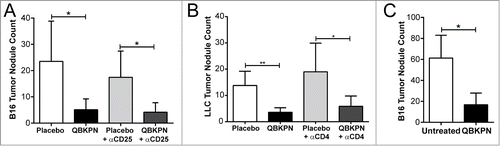
Figure 4. QBKPN stimulated an acute-like immune response, leading to innate cell recruitment into the lungs. (A) Principal component analysis of 31 cytokines/chemokines five hours after a single dose of placebo (circles) or QBKPN (squares) in the serum of mice. The first two principle components (PC) are shown. (B) Percentage of inflammatory monocytes (CD11b+CD115+Ly6CHI CCR2+) and (C) neutrophils (Ly6G+) as a percentage of CD45+ cells in the blood five hours after a single dose of placebo or QBKPN. (D) Proportion of immune cells in the lungs of mice in a B16F10 lung cancer model, administered placebo or QBKPN from day −10 until euthanasia at day 17. Upper pie charts show the proportion of B cells, T cells, NK cells and myeloid cells. Lower pie charts show the proportion of different myeloid cells. All percentages reflect the percent of CD45+ defined cells,Citation56 with NK cells (E) and interstitial macrophages (F) as a percentage of CD45+ defined cells individually shown. (G) Mean fluorescent intensity (MFI) ratio of CD80/CD206 of the CD11b+ lung cells (excluding neutrophils), in a B16F10 lung cancer model, administered placebo or QBKPN from day −10 until euthanasia at day 17. Mean +/− SD shown, n = 5–10 mice per group. *, P < 0.05; ***, P< 0.005 by Student's t-test.
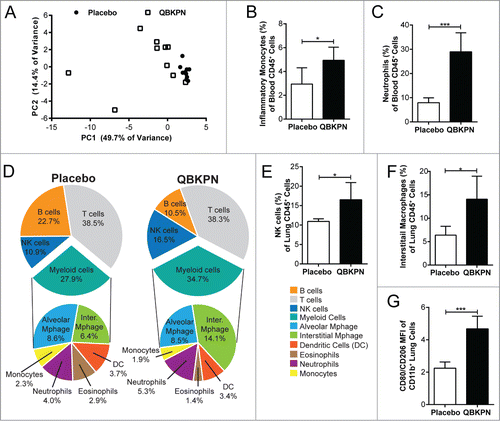
Figure 5. QBKPN anti-tumor efficacy required NK cells and the NKG2D pathway. (A) Mice were treated with anti-NK1.1 antibody or left untreated. The ratio of black pixels (surface B16F10 melanoma tumors) for mice administered with QBKPN to mice untreated from day −10 until euthanasia at day 14. (B) Quantitative RT-PCR of Granzyme B (GzmB), Granzyme A (GzmA) and Perforin 1 (Pfr1) in the lungs of mice administered placebo or QBKPN from day −10 until euthanasia at day 17, in the B16F10 model. (C) Granzyme B protein level in the lungs of mice administered placebo or QBKPN from day −10 until euthanasia at day 17, in the B16F10 lung cancer model. (D) Tumor levels in the lungs of wildtype and NKG2D−/− mice administered QBKPN or placebo from day −10 until euthanasia at day 11, in the B16F10 lung cancer model, measured by quantitative RT-PCR of Tyr. (E) Rae1+ cells as a percentage of red fluorescent protein (RFP) tagged LLC cells in the lungs of mice administered placebo or QBKPN from day −10 until euthanasia at day 15, in the LLC lung cancer model. (G) Rae1+ cells as a percentage of CD45+ cells in the lungs of mice administered QBKPN or placebo from day −10 until day 17, in the B16F10 lung cancer model. Mean +/− SD shown. N = 5 mice per group. *, P < 0.05; **, P < 0.01 by Student's t-test.
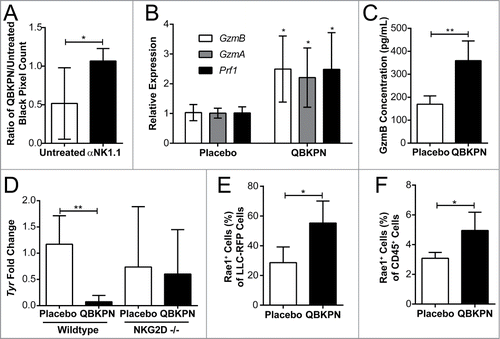
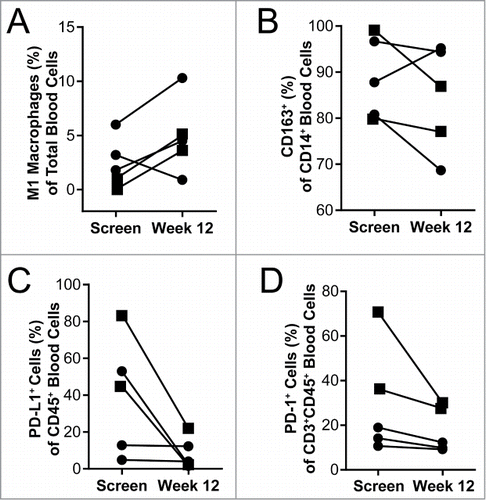
Figure 6. Blood immunology was changed in patients after 12 weeks of QBKPN treatment. (A) M1 macrophages (CD45+CD14+HLA-DR+CD86+) as a percentage of all cells in the blood of patients at screening and after 12 weeks of QBKPN treatment. (B) M2 macrophage marker expressing (CD163+) cells as a percentage of all CD14+ cells in the blood at screening and after 12 weeks of QBKPN treatment. (C) PD-L1+ cells as a percentage of all blood immune cells (CD45+) at screening and after 12 weeks of QBKPN treatment. (D) PD-1+ cells as a percentage of CD3+CD45+ cells in the blood at screening and after 12 weeks of QBKPN treatment. ▪, Neoplastic tumor bearing patients; •, pre-neoplastic tumor bearing patients.