Figures & data
Figure 1. mCD20-AcTaferon proof-of-principle. (A) General lay-out of an AcTaferon (AFN). The hIFNa2-Q124R, human IFNa2 with a Q124R point mutation, active on mouse cells but at a 100-fold lower level than murine IFN, is coupled to a sdAb module recognizing mCD20, hCD20, GFP or BcII10 via a GGS linker molecule. (B) Phospho-STAT1 as a read-out for IFN signaling in spleen CD19+ B lymphocytes isolated from mice treated 45 min earlier with i.v. PBS, serial dilutions of B cell targeted mCD20-AFN or untargeted GFP-AFN. (C) Proliferation of A20 cells treated in vitro for 72 hours with serial dilutions of mCD20-AFN or GFP-AFN. Results are expressed as percentage of cells versus untreated culture. (D) Growth of s.c. inoculated A20 tumors in syngeneic Balb/c mice after treatments with PBS, mCD20-mIFN (immunocytokine) or hCD20-mIFN (untargeted mIFN), mCD20-AFN ( = targeted), hCD20-AFN (untargeted control) or mCD20-hIFN (“sdAb-only” control). Arrows indicate treatment days, starting at day 6 after tumor inoculation. Shown is a representative experiment. Error bars represent mean ± s.e.m.; ***P < 0.001 and ****P < 0.0001 compared with PBS treated animals by two-way ANOVA with Dunnett's multiple comparison test (n = 5 mice per group).
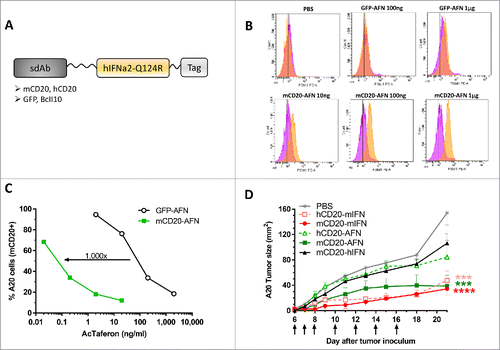
Figure 2. Targeted AcTaferon delivery to B16 tumors controls tumor growth without toxicity. (A) Growth of s.c. inoculated B16-mCD20+ tumors in syngeneic C57BL/6J mice after treatments with PBS, mCD20-mIFN (immunocytokine) or hCD20-mIFN (untargeted mIFN), mCD20-AFN (targeted) or hCD20-AFN (untargeted), or mCD20-hIFN as a negative control for “sdAb-only” effects. Shown is a representative experiment of 7 independent repeats (n = 5 mice per group), arrows indicate treatment days. (B) Seven independent experiments were pooled to plot the time necessary for each mouse to reach a tumor of 70 mm2 (total n = indicated in the legend). (C) Body weight changes of tumor-bearing mice treated with mCD20-targeted mIFN or AFN (n = 5). (D-I) Hematological analyses (platelet counts, mean platelet volume, red blood cell, neutrophil, monocyte and lymphocyte counts) in fresh EDTA-blood collected 1 day after the last treatment. ‘Naive mice’ are tumor-free, ‘tumor mice’ are tumor-bearing treated with PBS. All values depicted are mean ± s.e.m.; *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 compared with PBS treated animals unless otherwise indicated; by two-way ANOVA with Dunnett's multiple comparison test (A, C), one-way ANOVA with Dunnett's multiple comparison test (D-I) or log-rank test (B). Shown are representative results of 7 independent repeats (C-I).
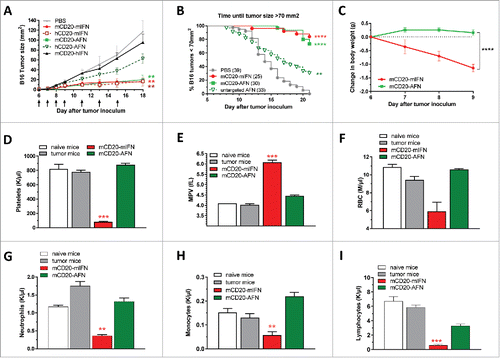
Figure 3. Partial lymphopenia due to mCD20-AcTaferon therapy is not required for antitumor efficacy. (A) Growth of s.c. inoculated B16-mCD20+ tumors in C57BL/6J mice after treatments with PBS, or different doses of mCD20-mIFN or mCD20-AFN. Shown is a representative experiment of 2 independent repeats (n = 5 mice per group). (B-D) Lymphocyte, neutrophil and platelet counts in fresh EDTA-blood collected 1 day after the last treatment. ‘Naive mice’ are tumor-free. All values depicted are mean ± s.e.m.; *P < 0.05, **P < 0.01 compared with PBS treated animals, by two-way ANOVA with Dunnett's multiple comparison test (A), or one-way ANOVA with Dunnett's multiple comparison test (B-D).
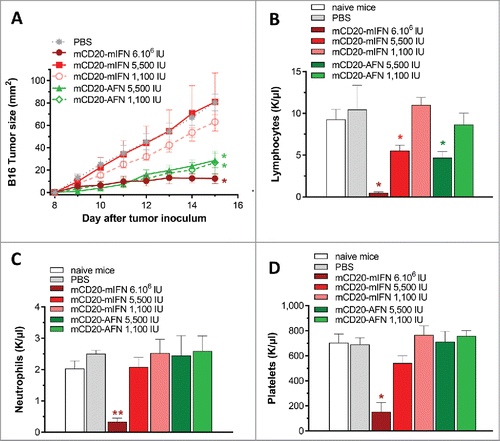
Figure 4. Antitumor efficacy of tumor-targeted AcTaferon depends on cDC1 and CTL. (A) Growth of B16-mCD20+ tumors in IFNAR1-deficient versus WT mice after 7 treatments with PBS or tumor-targeted mIFN or AFN (n = 5 mice per group). (B) Growth of B16-mCD20+ or B16-mCD20+-IFNAR−/− tumors in WT mice after 10 treatments with PBS or tumor-targeted AFN (n = 12 mice per group, pooled results of 2 different IFNAR−/− B16-mCD20+ clones). (C) Growth of s.c. inoculated B16-hCD20+ tumors in C57BL/6J mice after treatments with PBS, hCD20-AFN or mCD20-AFN. Shown is a representative experiment (n = 5 mice per group). (D) Lymphocyte counts in fresh EDTA-blood collected 1 day after the last treatment of mice represented in C. ‘Naive mice’ are tumor-free. (E) Growth of B16-mCD20+ tumors in Batf3−/− mice (lacking cDC1) and WT littermates after 6 treatments with PBS or mCD20-AFN (n = 7 mice per group). (F) Growth of B16-mCD20+ tumors in CD11c-IFNAR-deficient mice (lacking IFNAR in cDC1 and cDC2) and WT littermates after 5 treatments (n = 4 mice per group). (G) Growth of B16-mCD20+ tumors in CD8-depleted mice and controls after 6 treatments (n = 5 mice per group). (H) Growth of B16-mCD20+ tumors in CD4-IFNAR-deficient mice (lacking IFNAR in T lymphocytes) and WT littermates after 5 treatments (n = 4 mice per group). All results shown are a representative of two independent repeats. Shown are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 compared with PBS treated animals unless otherwise indicated; determined by two-way ANOVA with Dunnett's multiple comparison test.
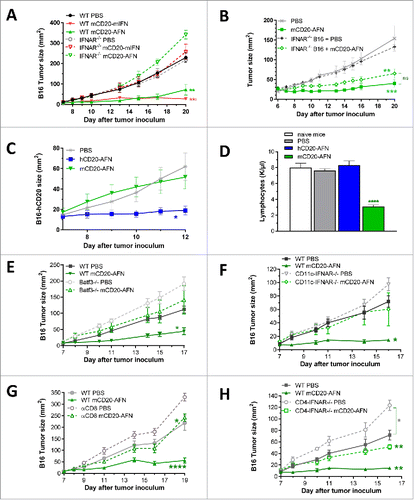
Figure 5. DC and CTL responses during AcTaferon treatments. (A) Flow cytometric profiling of the DC activation status in the tumor draining lymph node in response to AFN treatment. DCs were identified as CD3− CD19− Ly6C− CD11cint-hi MHCIIint-hi cells and subdivided into XCR1+ cDC1 and CD11b+ cDC2. Expression levels of PDL1, MHCII, CD80, CD86 and CD40 are displayed as MFI in the respective fluorescence channels. Results shown are a representative of two independent repeats (n = 5). (B) Flow cytometric analysis of CD3+ CD8+ T cell phenotype based on the expression of CD44 and CD62L was performed on tumor-draining lymph nodes of mice bearing B16 tumors, five days after perilesional delivery of the AFNs indicated in the figure legend (n = 3). Effector T cells were identified as CD44 high and CD62L low. (C) Flow cytometric analysis of Pmel-1 T cell proliferation in the tumor-draining lymph node in response to perilesional AFN treatment of B16-mCD20 tumor-bearing mice. Data show the percentage of T cells having undergone at least one division. (D-E) Flow cytometric analysis of CD3+ CD8+ T cell phenotype based on the expression of CD44 and CD62L was performed on B16 tumors, five days after perilesional delivery of the AFNs indicated in the figure legend (n = 3). Naive cells (D) were identified as CD44 low and CD62L high, effector T cells (E) as CD44 high and CD62L low. Shown are individual values (A) and mean ± s.e.m. of a representative experiment of at least 2 independent repeats (A-E); *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 compared with PBS treated animals unless otherwise indicated; by one-way ANOVA with Tukey's multiple comparison test.
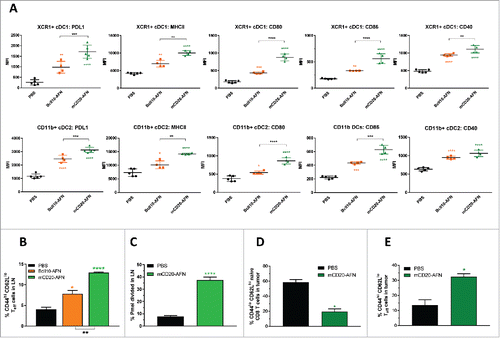
Figure 6. Tumor-targeted AcTaferon combination treatments eradicate tumors without toxicity. (A) Growth of s.c. inoculated B16-mCD20+ tumors in C57BL/6J mice, body weight loss (B), neutrophil counts (C) and mortality (D) after 8 treatments with PBS, tumor-targeted mCD20-mIFN or mCD20-AFN (n = 5 mice per group, shown is a representative experiment, arrows indicate treatment days). When indicated in the legends, tumors were also treated with dox(orubicine) every second day. (E) Growth of s.c. inoculated B16-mCD20+ tumors in C57BL/6J mice, body weight loss (F), platelet counts (G) and mortality (H) after 8 treatments with PBS, tumor-targeted mCD20-mIFN or mCD20-AFN (n = 5 mice per group, shown is a representative experiment, arrows indicate treatment days). When indicated in the legends, tumors were also treated with low-dose (0.6 μg/mouse) TNF every second day. (I-K) Growth of s.c. inoculated B16-mCD20+ tumors in C57BL/6J mice treated with PBS or mCD20-AFN. When indicated, treatment was combined with anti-PDL1 sdAb or a combination of Treg-depleting (TregΔ) anti-CTLA4 + anti-OX40 antibodies. Dividend/divisor in the figures indicates the number of tumor-free mice over the number of total mice at the day the experiment was ended, indicated in the X axis. For all figures, a representative experiment is shown (n = 4-6 per group), repeated at least twice. All values are mean ± s.e.m.; *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 compared with PBS treated animals unless otherwise indicated; by two-way ANOVA with Dunnett's multiple comparison test (A-B, E-F, I-K), one-way ANOVA with Dunnett's multiple comparison test (C, G) or log-rank test (D, H).
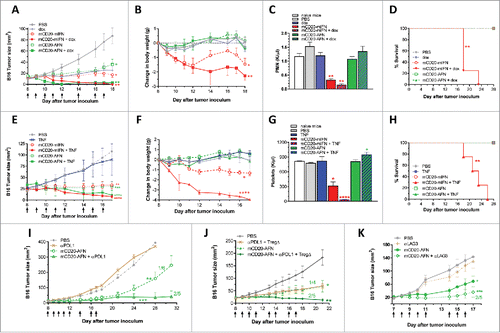
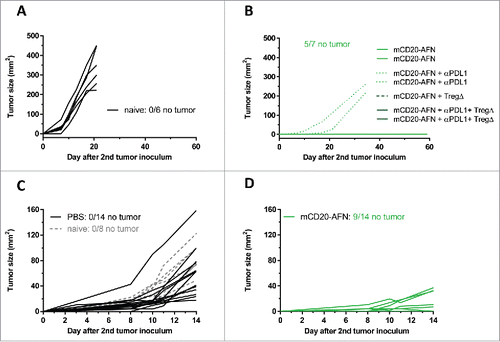
Figure 7. Tumor-targeted AcTaferon therapy provides immunity. (A) Growth of s.c. inoculated B16-mCD20+ tumors in naive C57BL/6J mice. (B) Growth of B16-mCD20+ tumors inoculated on the contralateral flank on day 35 in mice where complete eradication of the primary tumor was achieved thanks to specific treatments (day 7–17) indicated in the figure legend. Tumor growth was evaluated for 60 days after the second tumor inoculation. (C) Growth of s.c. inoculated B16-mCD20+ tumors in naive C57BL/6J mice, or in mice where the primary tumor was treated with PBS (day 5–10). (D) Growth of B16-mCD20+ tumors inoculated on the contralateral flank on day 12 in mice that received p.l. treatment for their primary tumor with mCD20-AFN on days 5–10. The experiment was ended 14 days after the second tumor inoculation (26 days after the primary tumor inoculation). Tumor growth of individual mice are plotted, the number of mice that remained tumor-free for the duration of the experiment (60 days for A-B and 14 days for C-D) is indicated in each figure.