Figures & data
Figure 1. Increased suppressive phenotype of tumor-infiltrating Tregs. Foxp3-GFP mice were injected on day 0 with 1 × 106 TC-1 cells, and 25 days later, the mice were sacrificed and cell suspensions were prepared from the spleens (SpT), tumor-dLN (dLN) and tumors, stained and analyzed by flow cytometry, and compared with the LN (LNN) and spleen (SpN) from naive mice. (A-D) The surface expression of PD-1, CD39, CD73, CD103, ICOS, GITR, PD-L1 and CTLA-4 (filled grey line) by CD4+ Foxp3+ Tregs purified from the indicated organs is shown. Labeling with isotype controls is represented by black lines. Data from one representative experiment are shown in A and C, whereas the geometric mean fluorescence intensity (MFI) ± SEM is shown in B and D. The results represent the cumulative data from 4 independent experiments (n = 8–9 mice per group). * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001 as determined by the Mann-Whitney test.
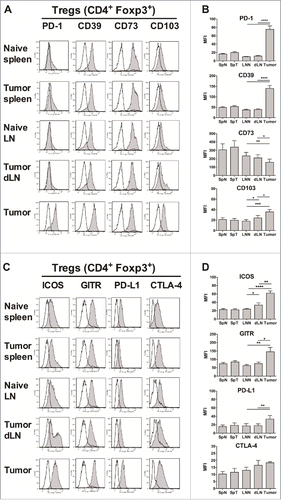
Figure 2. Anti-PD-1 antibody treatment does not enhance the therapeutic efficacy of the CyaA-E7 vaccine. C57 BL/6 J mice were injected with 6 × 105 TC-1 cells on day 0, and after 14 (A) or 24 (B) days were i.v. immunized with CyaA-E7 (50 µg/mouse) and CpG-B-DOTAP (left panels) or received PBS (right panels). On day 13 (A) or 23 (B) and then every 3 days after vaccination, the mice were left untreated (upper panels) or received either i.p. injection of anti-PD-1 (middle panels) or isotype control antibodies (lower panels) for a total of 4 injections (250 µg/mouse/injection on days 13, 17, 20 and 23 in A or days 23, 27, 30 and 33 in B). The results represent the cumulative data from 2 independent experiments (n = 14–15 mice per group).
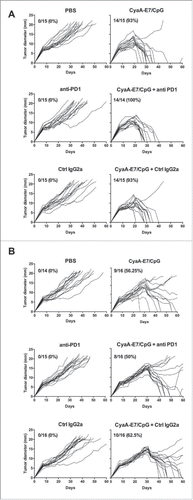
Figure 3. Functional analysis of tumor-infiltrating T-cells. Foxp3-GFP mice were injected on day 0 with 1 × 106 TC-1 cells, and the spleens, dLN and tumors were processed 25 days later to obtain cell suspensions. The spleens and lymph nodes from naive mice were used as controls. CD4+ Foxp3-GFP+ Tregs and Foxp3-GFP− Teffs were FACS sorted, and then total RNA was purified from the sorted cells and reverse-transcribed. The corresponding cDNA material was then subjected to quantitative PCR micro-arrays with primers specific to Foxp3 (A), RORγc (B), T-bet (C) and GATA-3 (D). The results represent the mRNA expression normalized to house-keeping genes and are expressed as the mean arbitrary units ± SD from 2 independent experiments in which cells from each organ were pooled from 5–6 mice per group. One-way ANOVA was performed separately for each subset to compare its gene expression in the different tissues. *p<0.05 was obtained for T-bet expression in both Tregs and Teffs (C) and **p<0.01 for GATA-3 expression in Tregs (D). (E-F) The cell suspensions were stained and analyzed by flow cytometry. The intracellular expression of T-bet in Tregs and Teffs is shown in E, while F shows the mean MFI ± SEM of cumulative data from 3 independent experiments (n = 5–6 mice per group). * p< 0.05 as determined by the Mann-Whitney test in F. (G-H) After AutoMacs CD90+ cell enrichment, CD4+ Foxp3-GFP+ Tregs and Foxp3-GFP− Teffs were FACS sorted, mixed together at different ratios, and cultured in anti-CD3-coated plates (5 µg/mL) for 72 hours. Cell proliferation was determined by [3H]-thymidine incorporation (G), and IFNγ was measured in culture supernatants (H). The results are expressed as the mean ± SEM from 2 independent experiments in which cells from 8 mice were pooled.
![Figure 3. Functional analysis of tumor-infiltrating T-cells. Foxp3-GFP mice were injected on day 0 with 1 × 106 TC-1 cells, and the spleens, dLN and tumors were processed 25 days later to obtain cell suspensions. The spleens and lymph nodes from naive mice were used as controls. CD4+ Foxp3-GFP+ Tregs and Foxp3-GFP− Teffs were FACS sorted, and then total RNA was purified from the sorted cells and reverse-transcribed. The corresponding cDNA material was then subjected to quantitative PCR micro-arrays with primers specific to Foxp3 (A), RORγc (B), T-bet (C) and GATA-3 (D). The results represent the mRNA expression normalized to house-keeping genes and are expressed as the mean arbitrary units ± SD from 2 independent experiments in which cells from each organ were pooled from 5–6 mice per group. One-way ANOVA was performed separately for each subset to compare its gene expression in the different tissues. *p<0.05 was obtained for T-bet expression in both Tregs and Teffs (C) and **p<0.01 for GATA-3 expression in Tregs (D). (E-F) The cell suspensions were stained and analyzed by flow cytometry. The intracellular expression of T-bet in Tregs and Teffs is shown in E, while F shows the mean MFI ± SEM of cumulative data from 3 independent experiments (n = 5–6 mice per group). * p< 0.05 as determined by the Mann-Whitney test in F. (G-H) After AutoMacs CD90+ cell enrichment, CD4+ Foxp3-GFP+ Tregs and Foxp3-GFP− Teffs were FACS sorted, mixed together at different ratios, and cultured in anti-CD3-coated plates (5 µg/mL) for 72 hours. Cell proliferation was determined by [3H]-thymidine incorporation (G), and IFNγ was measured in culture supernatants (H). The results are expressed as the mean ± SEM from 2 independent experiments in which cells from 8 mice were pooled.](/cms/asset/93eb3049-31f3-4ff2-a633-900a8b8e0ef7/koni_a_1404213_f0003_b.gif)
Figure 4. The intratumoral recruitment of CD8+ T lymphocytes is increased in MHC class II-deficient mice. (A) Wild-type C57BL/6J (WT; black lines) and MHC-II KO mice (green lines) were injected on day 0 with 6 × 105 TC-1 cells, and tumor growth was followed every 2–3 days. The number and percentage of tumor-free mice on day 70 compared with the total number of animals injected are shown. (B-E) Wild-type C57BL/6J and MHC-II KO mice were injected on day 0 with 6 × 105 TC-1 cells, and on day 25, cell suspensions were prepared from spleens, dLN and tumors and analyzed by flow cytometry. The spleens and lymph nodes from naive mice were used as controls. The numbers of lymphocyte subsets and their percentages within the total CD45+ in spleen (B and C), in LN (D and E), and in tumors (F and G), respectively are shown. B-G show the mean ± SEM of cumulative results from 3 independent experiments (n = 6–7 mice per group). *p < 0.05, ** p < 0.01 and ***p < 0.001 as determined by Mann-Whitney's test between each lymphoid subset in WT vs MHC-II KO mice for each organ.
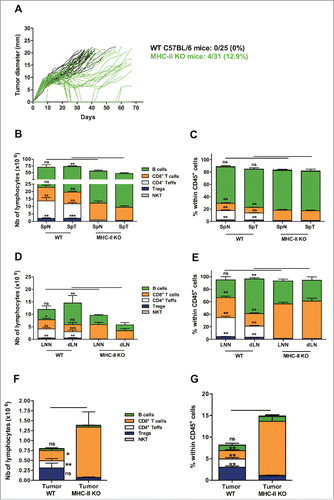
Figure 5. Similar activation status of Tregs in WT and MHC-II KO mice. WT C57BL/6J and MHC-II KO mice were injected on day 0 with 6 × 105 TC-1 cells, and cell suspensions were prepared after 25 days from spleens, dLNs and tumors and analyzed by flow cytometry. The spleens and lymph nodes from naive mice were used as controls. The surface expression of CD44, CD62 L, PD-1, CD103, ICOS and GITR on Tregs from the indicated organs is shown in A and C. Filled grey lines represent WT mice, open red lines represent MHC-II KO mice, while labeling with isotype controls is represented by black lines. Data from one representative experiment are shown. The MFI ± SEM shown in B and D represents cumulative results from 4 independent experiments with n = 7–11 mice per group (WT: grey histograms and MHC-II KO mice: red histograms). ** p < 0.01 and *** p < 0.001 as determined by the Mann-Whitney test.
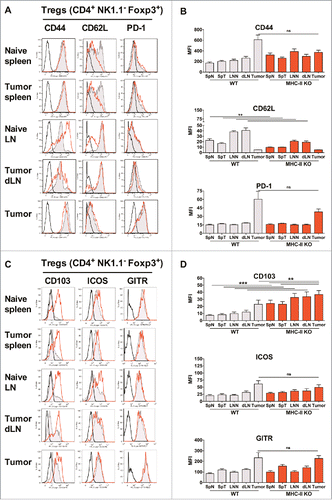
Figure 6. CD8+ T lymphocytes are activated in MHC-II KO mice and recruited to the tumor, despite the absence of CD4+ T-cells. WT C57BL/6J and MHC-II KO mice were injected on day 0 with 6 × 105 TC-1 cells, and cell suspensions were prepared after 25 days from spleens, dLN and tumors and analyzed by flow cytometry. The spleens and lymph nodes from naive mice were used as controls. The surface expression of CD44, CD62L, PD-1, ICOS, GITR and CD103 on CD8+ T-cells purified from the indicated organs is shown in A and C. Filled grey lines represent WT mice, open red lines represent MHC-II KO mice, while labeling with isotype controls is represented by black lines. Data from one representative experiment are shown. The MFI ± SEM shown in B and D (WT: grey histograms and MHC-II KO: red histograms) represents cumulative results from 4 independent experiments (n = 7–11 mice per group). * p < 0.05, ** p < 0.01 and *** p < 0.001 as determined by the Mann-Whitney test.
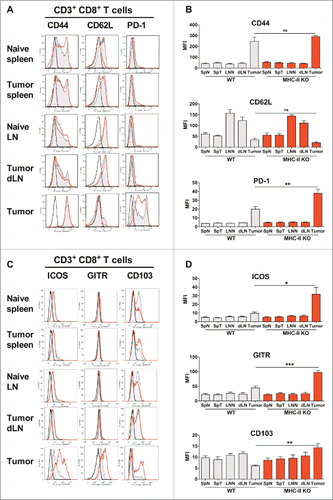
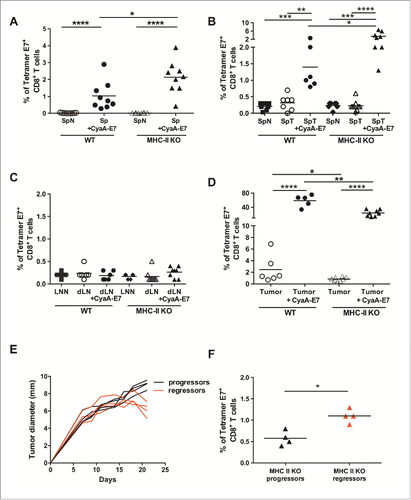
Figure 7. MHC-II KO mice develop strong specific anti-tumor CD8+ T-cell responses after vaccination or during tumor growth, independently of CD4+ T-cell help. (A) Naive WT and MHC-II KO mice were vaccinated on day 0 with either CyaA-E7 (50 µg/mouse) and CpG-B-DOTAP (30 and 60 µg/mouse, respectively) or PBS. On day 7, the mice were killed, cell suspensions were prepared from their spleens and the percentages of tetramer-E7+ CD8+ T-cells were analyzed by flow cytometry. Cumulative results from 3 independent experiments are shown (n = 9 mice per group). (B-D) WT and MHC-II KO mice were injected on day 0 with 6 × 105 TC-1 cells, cell suspensions were prepared from tumors after 20 days, and the percentages of tetramer-E7+ CD8+ T-cells were analyzed by flow cytometry. Mice vaccinated on day 13 were used as a positive control. The results represent cumulative data from 2 independent experiments. The percentages of tetramer E7-positive CD8+ T-cells in spleen (B), LN (C) (n = 6–8 mice per group) and tumor (D) (n = 5–8 mice per group) are shown. (E-F) MHC-II KO mice were injected on day 0 with 6 × 105 TC-1 cells. In E, the red curves represent tumor growth up to day 20 in mice that started to reject their tumor (regressor mice), while the black curves represent tumor growth in mice with growing tumors (progressor mice). (F) Mice were killed at day 20, and the percentages of tetramer E7-positive CD8+ T-cells were analyzed in the tumors of progressor and regressor MHC-II KO mice (n = 4 mice per group). * p< 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001 as determined by the Mann-Whitney test.