Figures & data
Figure 1. Schematic of CAR structures containing the CD5-directed variable lymphocyte receptor (VLR) or single chain variable fragment (scFv). (A) Second generation CAR structures with CD28 containing a scFv (left) or VLR (right) as the antigen recognition domain. (B) Schematics of the bicistronic transgene sequences used for expressing enhanced green fluorescent protein (eGFP) and the CD5-CARs using a P2 A sequence. It includes a 5′ long terminal repeat (LTR), human ubiquitin C promoter (hUBC), eGFP sequence, P2 A sequence, an interleukin-2 signal peptide (IL-2 SP), the CD5-VLR (top) or CD5-scFv (bottom), a myc epitope tag, the CD28 region, the CD3ζ intracellular domain and a 3′ LTR.
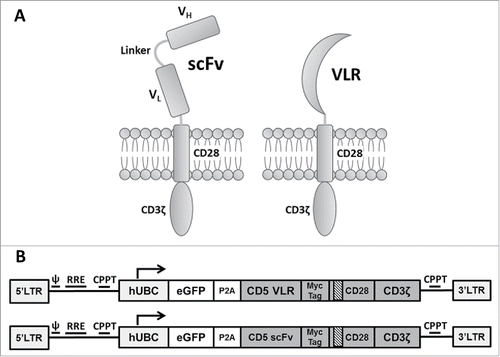
Figure 2. NK-92 cell mediated cytotoxicity against a CD5-positive T-ALL cell line using CD5-CARs. (A) NK-92 cells were transduced with the eGFP-P2 A-CD5-scFv-CAR lentiviral vector and sorted for GFP expressing cells. After two rounds of sorting an enriched population of CAR-expressing NK-92 cells was generated with 99% eGFP expression. (B) Western blot using anti-CD3ζ antibody on whole cell lysates of NK-92 cells shows the presence of CD5-VLR-CAR and CD5-scFv-CAR protein in the sorted and expanded cells. (C & D) Both CD5-CAR expressing NK-92 cells were mixed with CD5-positive target cells, Jurkat and MOLT-4, at various Effector: Target ratios and the percent cytotoxicity was measured by flow cytometry. CD5-CAR modified NK-92 cells showed a significantly greater cytotoxicity (p < 0.01) against the CD5-positive Jurkat and MOLT-4 cells when compared to unmodified NK-92 cells in a 4 hour assay. (E) No increase in cytotoxicity is seen when CD5-CAR NK-92 cells are cultured with CD5-negative 697 cells. Errors bars represent standard deviations.
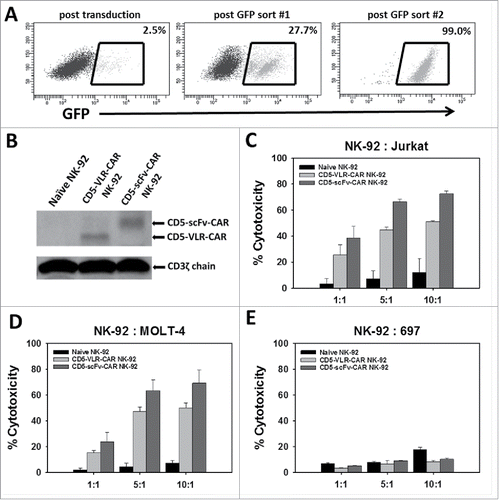
Figure 3. Initial comparison of VLR- and scFv-based CD5-CARs. (A) Jurkat T cells were transduced with lentiviral vectors encoding either an scFv- or VLR-based CD5-CAR with co-expression of eGFP. Schematic of the Jurkat T cell activation assay shows time points for measurement of T-cell activation and Western blot analysis. (B) Activation measured by surface CD69 expression four days after transduction increased as the amount of viral vector increased. Greater activation was observed in the CD5-VLR-CAR Jurkat group. (C) The percentage of activated cells was compared to the vector copy number (VCN) obtained for each transduced population of cells. The inset to the figure defines each group. (D) CD69 expression was measured 4 and 12 d after transduction, which showed activation decreased over time in both CD5-CAR expressing Jurkat T cell groups. Errors bars represent standard deviations.
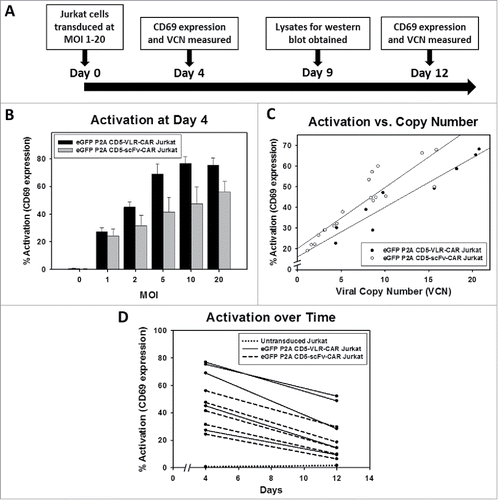
Figure 4. CD5 knockout in Jurkat T cells using CRISPR-Cas9 genome editing. (A) CD5 expression, measure by flow cytometry, in Jurkat T cells five days following mock transfection or transfection with plasmid encoding Cas9 and one of three different gRNA target sequences. Histogram plots for CD5 expression in mock transfected and transfected Jurkat T cells are shown along a single axis. (B) Overlay image of histogram plots of CD5 expression in naïve Jurkat T cells and flow-sorted CD5-negative Jurkat T cells that were transfected with the CD5-CRISPR gRNA #2. (C) Representative Sanger sequencing traces from naïve (top left) and sorted CD5-edited (top right) Jurkat T cell genomic DNA PCR amplified for CD5. TIDE analysis of the frequency of indels within the CD5 gene after the predicted break-site generated by Cas9 (D). Results show 77% CD5-negative cells were edited with 27% having a −1 deletion.
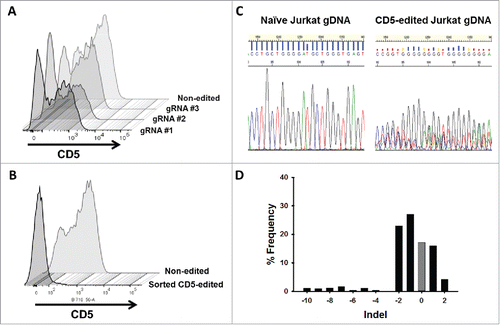
Figure 5. CD5-edited CD5-CAR-modified Jurkat T cells have reduced self-activation and increased CD5-CAR expression. Naïve (white) and CD5-edited Jurkat T cells (black) were transduced with eGFP-P2 A-CD5-VLR-CAR, eGFP-P2 A-CD5-scFv-CAR or control eGFP-P2 A-BCL-VLR-CAR lentiviral vectors at MOIs 1, 10 and 20. Polybrene was not used during transduction, which provided a greater separation in transduction efficiency between MOIs of 1 and 10. Experiments were performed with replicates of three or greater (error bars are generated using the standard deviation from the mean) except for CD5-scFv-CAR at MOI 10 and 20, which were done in duplicate, providing the difference of the mean. (A) Transduction efficiency, measured by eGFP-positive cells, of each CAR vector at MOIs 1, 10 and 20 in both populations of Jurkat T cells. (B) CD5 expression in both populations of Jurkat T cells transduced with each CAR vector at each MOI. (C) Activation was measured by monitoring CD69 expression and transduction efficiency was measured by eGFP expression. A correlation exists between activation and eGFP expression in CD5-CAR-transduced Jurkat T cells. Non-edited CD5-CAR-modified cells have increased T-cell activation compared to CD5-edited CD5-CAR-modified cells. (D) Western blots on whole cell lysates showing CD3ζ expression in non-edited Jurkat T cells (left) and CD5-edited Jurkat T cells (right) when transduced with the VLR-CAR vector. Endogenous CD3ζ is represented by the 18 kDa bands and CD3ζ in the CAR construct is represented by the 48, kDa band in the CD5-VLR-CAR construct. eGFP, CD5 and CD69 surface expression were measured by flow cytometry.
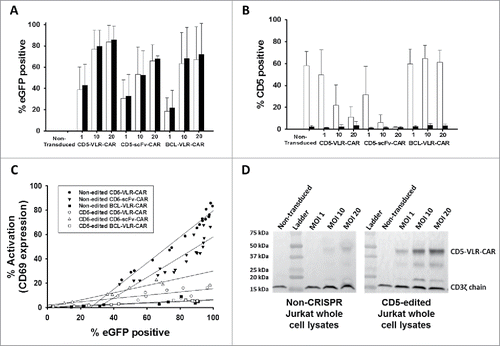
Figure 6. CD5-edited CD5-CAR-modified effector cells in culture with naïve target T cells stimulates effector cell activation and target cell down-regulation of CD5. Naïve and CD5-edited Jurkat T cells were transduced with eGFP-P2A-CD5-scFv-CAR or eGFP-P2A-CD5-VLR-CAR lentiviral vectors at MOI 5. Polybrene was not used during transduction. Target naïve Jurkat T cells were labeled with VPD450. On day five post-transduction, effector cells were cultured with labeled target cells at E:T ratios 2:1, 1:1 and 1:5. The cells were analyzed by flow cytometry 24 hours later. White bars signify non-edited effector cells; black bars signify CD5-edited effector cells. Experiments were performed with three replicates and error bars represent standard deviation from the mean. (A and B) Percent of baseline CD5 expression in target Jurkat T cells cultured with non-edited and CD5-edited effector Jurkat T cells expressing the CD5-scFv-CAR (A) or the CD5-VLR-CAR (B). CD5 expression in target cells cultured alone (gray bar) was used as baseline and set at 100%. (C and D) T-cell activation of non-edited and CD5-edited effector Jurkat T cells expressing the CD5-scFv-CAR (C) or the CD5-VLR-CAR (D) when cultured alone and in culture with target Jurkat T cells.
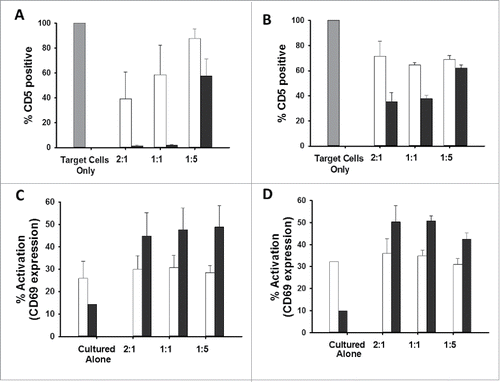
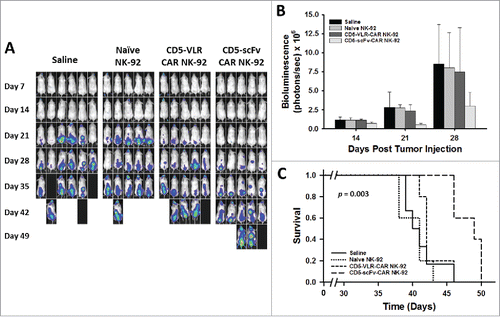
Figure 7. CD5-scFv-CAR NK-92 cells are superior to CD5-VLR-CAR NK-92 cells in delaying disease progression and improving survival in a T-ALL xenograft mouse model. (A) NSG mice were injected with 2 × 106 luciferase-expressing Jurkat T cells intravenously on day 0. Treatment was started 7 d after tumor injection with each mouse receiving a total of 4 treatments on days 7, 11, 14 and 18. Mice were assigned to 4 different treatment groups – saline, naïve NK-92, CD5-VLR-CAR NK-92 or CD5-scFv-CAR NK-92. A dose of 107 cells per mouse were administered at each treatment time. Bioluminescence imaging was performed every seven days to monitor tumor burden. (B) Total bioluminescence from Jurkat T cells on days 14, 21, and 28 post tumor injection. A significant decrease (p < 0.05) in tumor burden is observed in the CD5-scFv-CAR NK-92 group. Errors bars represent standard deviations. (C) Kaplan-Meier survival curves showing overall survival. Mice treated with CD5-scFv-CAR NK-92 cells showed significant increased survival (p < 0.05) when compared to all other treatment groups. Mice treated with CD5-VLR-CAR NK-92 cells did not have a significant advantage over saline and naïve NK-92 treatment groups.