Figures & data
Figure 1. xCT structure and VLP production. (A) Human xCT contains six extracellular domains that range from 2 aa (ECD5) to 31 aa (ECD4) (NCBI Reference Sequence: NP_055146.1). The human ECDs exhibit high homology to mouse with ECD6 showing 100% identity. (B) Production of xCT VLPs. The single chain MS2 coat protein (CP) was synthesized to contain an individual xCT ECD displayed in the AB loop domain to more closely mimic native xCT structure. Plasmids were introduced into cells by transformation where MS2 CP self assembles into VLPs. Bacteria were lysed and VLPs were purified from the soluble protein fraction. The xCT VLPs differ from wild-type MS2 VLPs only in the xCT ECD peptide displayed in the surface exposed AB loop.
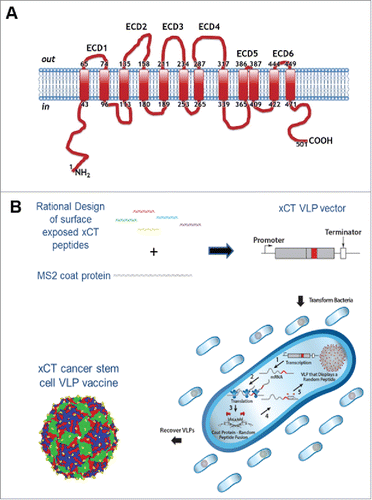
Figure 2. AX09-0M6 induced strong antibody responses. (A) Peptide ELISA of sera from treated BALB/c mice were serially diluted and incubated with xCT ECD6 peptide coated wells. End-point titers were determined by the highest dilution that was 2-fold over background. No titer was observed with control sera. Each dot represents an individual animal treated with AX09-0M6 and results are from 3 independent experiments. (B) AX09-0M6 sera from panel A were analyzed for relative amounts of different IgG isotypes. The relative ratio of each IgG subclass was calculated for each animal by taking the average OD450 value from a given IgG subclass (IgGx) divided by the average OD450 from total IgG. (C-F) FACS analysis of tumorspheres derived from TUBO (C), 4T1 (D), HCC-1806 (E) or MDA-MB-231 (F) cells incubated with 50 µg/ml IgG purified from sera of MS2 wt or AX09-0M6-vaccinated mice, or with a commercial anti-xCT Ab. Representative histograms are shown. Graphs show the mean ± SEM of the percentage of positive cells set on the base of FITC-conjugated secondary Ab staining and of mean fluorescence intensity from 3 independent experiments. *, P < 0.05; **, P < 0.01, ***, P < 0.001, Student's t-test .
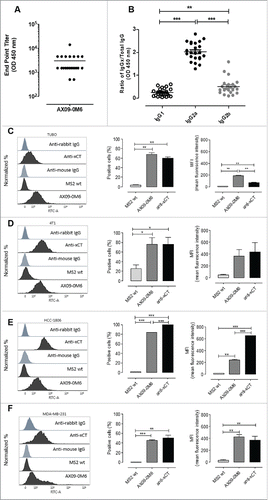
Figure 3. AX09-0M6-induced antibodies target tumorspheres. Representative immunofluorescence images of TUBO (A), 4T1 (B), HCC-1806 (C) and MDA-MB-231 (D)-derived tumorspheres incubated with IgG (50 μg/ml) purified from sera of BALB/c mice vaccinated with MS2 wt or AX09-0M6. The specific signal (green) was detected with an Alexa Fluor488-conjugated anti-mouse secondary antibody. Nuclei were counterstained with DAPI (blue). Magnification 40X, Scale bar, 40 µm **, P < 0.01, Student's t-test.
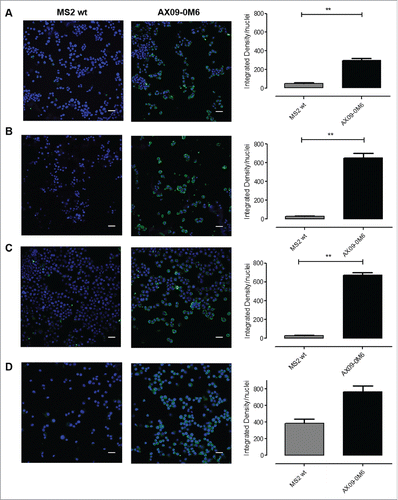
Figure 4. Vaccine-induced antibodies target CSC and affect self-renewal and ROS flux. TUBO (A), 4T1 (B), HCC-1806 (C) and MDA-MB-231 (D)-derived tumorspheres were incubated for 5 days with medium, IgG (50 μg/ml) purified from sera of BALB/c mice vaccinated with MS2 wt or AX09-0M6, or with SASP (50 μM). i) Sphere generating ability reported as tumorsphere number/103 plated cells. ii) Sphere diameter measured with the AxioVision 4.8 software. FACS analysis of iii) Aldefluor positivity reported as percentage of positive cells and of iv) ROS production, reported as DCF MFI. All graphs show mean ± SEM from at least three independent experiments. *, P < 0.05; **, P < 0.01, ***, P < 0.001, Student's t-test.
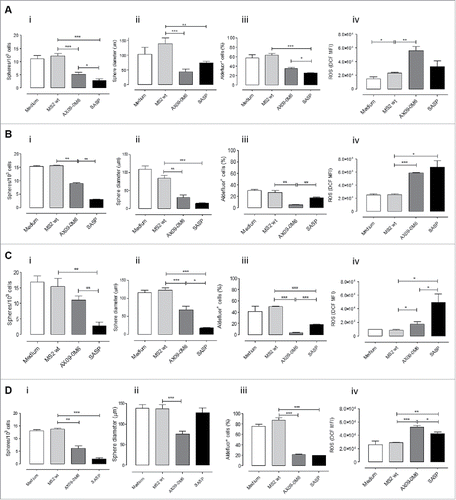
Figure 5. No signs of toxicity in the spleen and brain of AX09-0M6 treated mice. (A-C) FACS analysis of SPC from mice left untreated (white bars) or vaccinated with MS2 wt (black bars) or AX09-0M6 (gray bars). A) Percentage ± SEM of CD11c+ dendritic cells, Ly6G+Ly6C+ neutrophils and F4/80+ macrophages among the CD45+ cell population. (B) Percentage ± SEM of xCT+ CD11c+ dendritic cells, Ly6G+Ly6C+ neutrophils and F4/80+ macrophages among the CD45+ cell population. (C) Percentage ± SEM of xCT+ HLA class II+ M1, HLA class II+ CD206+ M1/M2, CD206+ M2 and HLA class II− CD206− M0 F4/80+ macrophages among the CD45+ cell population. (D-F) F40/80 immunohistochemical staining in brain from untreated (D), MS2 wt (E) and AX09-0M6 (F) representative mice; microglial cell processes are stained in brown. (20X, scale bar 100 µm).
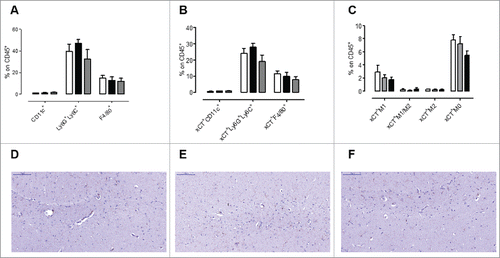
Figure 6. AX09-0M6 attenuates metastases in vivo affecting the immune infiltrate and induces antibodies stimulating ADCC. (A) Mice were treated with MS2 wt or AX09-0M6 in the absence of exogenous adjuvant. One week after final administration, P2 tumorspheres derived from TUBO cells were injected into the tail vein of treated mice. 20 days after cell challenge, the lungs were removed, sectioned and the number of micrometastases was determined. Each dot represents a single animal and is the average number of lung metastases from at least 2 sections. Results shown are the aggregate from 3 independent experiments. *, P = 0.0293; **, P = 0.0025, Mann Whitney test. (B-D) Cytofluorimetric analysis of immune infiltrates in lungs of mice left untreated (white bars) or vaccinated with MS2 wt (black bars) or AX09-0M6 (gray bars). (B) Graph shows the percentage ± SEM of CD45+ cells expressing the markers of T (CD3+CD49b−), NK (CD3−CD49b+), γδ T (CD3+γδ+), and NKT (CD3+CD49b+) cells. (C) Percentage ± SEM of CD4+ or CD8+ cells among the CD45+CD3+ T cell population. (D) Percentage ± SEM of Ly6G+Ly6C+ neutrophils, Ly6G−Ly6C+ monocytic MDSC and F4/80+ macrophages among the CD45+CD11b+ myeloid cell population. Three independent experiments were performed and a representative one is shown. E) ADCC assay was performed using 4T1 target cells incubated with 1:50 pooled sera from vaccinated mice (AX09-0M6, gray bars; MS2 wt, black bars and untreated, white bars) and SPC effector cells at different effector/target cells ratios (200:1, 100:1, and 50:1). Results shown are the mean ± SEM of the percentage of ADCC. *, P < 0.05; **, P < 0.01, ***, P < 0.001, Student's t-test.
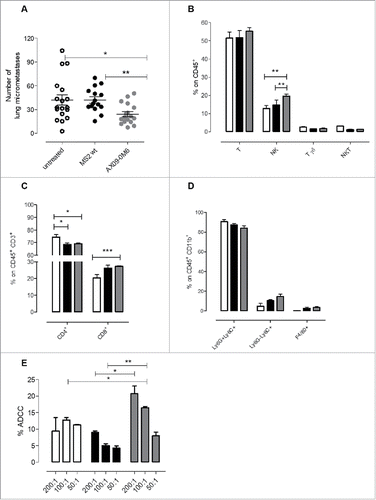
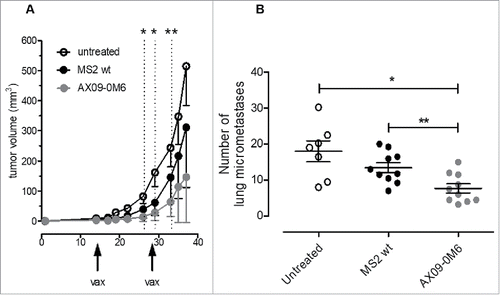
Figure 7. AX09-0M6 is efficacious in preventative and therapeutic models of aggressive breast cancer. (A) Passage 1 tumorspheres derived from 4T1 cells were injected into the inguinal mammary fat pad. When the tumors reached 1 – 1.5 mm in diameter, mice were treated with MS2 wt and AX09-0M6, and boosted 14-days later. Primary tumor diameters were measured at the indicated time-points and tumor volume was calculated. Results shown are the average tumor volume from two independent experiments and the error bars represent the standard deviation of the mean. AX09-0M6 vs MS2 wt: *, P < 0.05; **, P < 0.005; Student's t test. AX09-0M6 vs untreated: P < 0.005 from week 26 to 33; P < 0.05 from week 35; Student's t-test. (B) Mice from A were euthanized 40 days after tumor challenge and the number of micrometastases was enumerated. Each dot represents a single animal and is the average number of lung metastases from at least 2 sections. *, P = 0.0126; **, P = 0.0046, Mann Whitney test. Results shown are the aggregate from 2 independent experiments.