Figures & data
Figure 1. HER2-VLP vaccine design and characterization. A. The HER2 extracellular domain (ECD) was genetically fused at the N-terminus to SpyCatcher (SpyC). TM = trans membrane, ICD = intracellular domain. Arrows indicate HER2 subdomains targeted by licensed mAbs, pertuzumab and trastuzumab B. Schematic showing the HER2-VLP vaccine development process. The Spytagged VLP subunit and the SpyCatcher-HER2 antigen are separately expressed and purified, and then mixed. The tag/catcher conjugation insures a directional, high-density ‘virus-like’ display of HER2 on the surface of the VLPs. C. Reduced SDS-PAGE gel showing individual vaccine components. M = Size marker. Lane 1 contains uncoupled Spytagged VLP. Lane 2 contains subunit SpyCatcher-HER2. Lane 3 shows the resultant SpyCatcher-HER2:VLP conjugates after incubation of the components. Specifically, the lane contains both unconjugated VLP subunit (∼18.5 kDa) and SpyCatcher-HER2 antigen (marked by asterisk) (∼92 kDa) as well as tag/catcher mediated conjugates consisting of a VLP subunit bound to either one or two SpyCatcher-HER2 antigens. Lane 4 shows the final HER2-VLP vaccine product after residual uncoupled SpyCatcher-HER2 has been removed by dialysis. D. Transmission electron microscopy of HER2-VLP showing uniform, non-aggregated particles of approximately 66 nm.
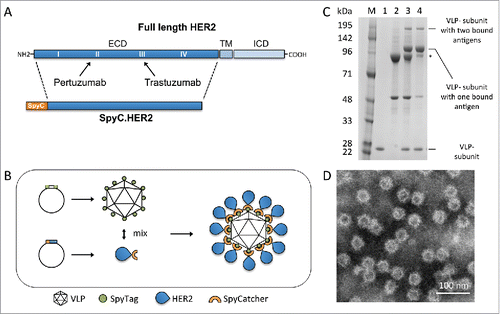
Figure 2. Therapy of HER2 mammary carcinomas with the HER2-VLP vaccine. Tumors expressing HER2 were established in groups of five female FVB mice by s.c. injection of MAMBO89 cells (A-C) or of mammary carcinoma fragments (D-F), see Materials and Methods for details. HER2-VLP vaccinations were administered in the tibialis anterior muscle every second week starting either 5 weeks after cell injection or 2 weeks after fragment implantation. (A, D) tumor growth curves, each line represents one mouse. (B, E) Kaplan-Meier analysis of data shown in panels A and D. Each line represents the proportion of mice that were either tumor-free or bore tumors smaller than 1 cm3; Significance of difference by the Mantel-Haenszel test: p < 0.01 (B), p = 0.01 (E). (C, F) Serum concentration of Abs against human HER2 extracellular domain, as measured by a specific ELISA; significance of difference by the non-parametric Mann Whitney Rank Sum Test: p<0.01.
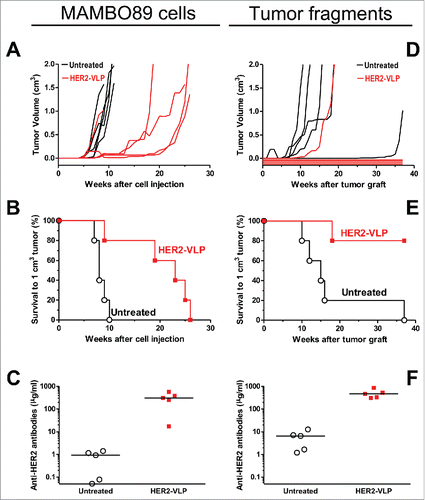
Figure 3. Prevention of HER2-driven mammary carcinogenesis by HER2-VLP vaccination. Groups of three transgenic mice carrying a human Delta 16 HER2 transgene were vaccinated with HER2-VLP or with monomeric SpyCatcher-HER2 plus untagged VLPs (labeled in graph as Untagged) (A-C). Groups of 7–11 F1 mice carrying a Delta16 and a full-length HER2 transgene were vaccinated with HER2-VLP or with an electroporated, HER2-encoding plasmid, pHuRT (D-F). I.m. vaccinations with HER2-VLP or pHuRT started at 5–8 weeks of age, when mice were healthy and tumor-free, and were administered every second week. (A, D) Tumor-free survival curves (Kaplan-Meier); the survival of mice vaccinated with HER2-VLP was significantly longer than that of all other groups by the Mantel-Haenszel test: p<0.001 (A, D). (B, E) Mean number of mammary carcinomas per mouse (tumor multiplicity). HER2-VLP vaccinated mice had significantly fewer tumors than untreated mice (at all time points beyond 22 weeks of age) and pHURT vaccinated mice (at time points between 11 and 24 weeks) (p <0.05; Student's t test.) (C, F) Kinetics of anti-human HER2 Abs in mouse serum measured by a specific ELISA. Each point represents the mean ± SEM of 3–13 individual sera of different mice. Antibody concentrations elicited by HER2-VLP were significantly higher than those elicited by pHuRT at 2, 4 and 6 weeks, p<0.05 (Student's t test).
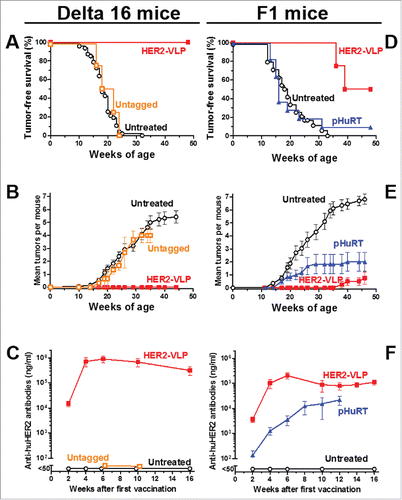
Figure 4. Antibodies elicited by the HER2-VLP vaccine bind the HER2 extracellular domain on the surface of human tumor cells. (A) Flow cytometry profiles of cells expressing known amounts of HER2, from completely negative (SJ-RH4) to highly positive (SK-OV-3) cells, stained with the serum of one representative F1 mouse vaccinated with HER2-VLP. (B) Comparison of staining either with sera of 3–4 individual F1 mice vaccinated with HER2-VLP (closed diamonds) or with anti-HER2 mAb MGR2 (open diamonds). Fluorescence intensities measured by flow cytometry were normalized by the concentration of IgG against HER2 measured by ELISA in each serum (MFI/HER-2 IgG).
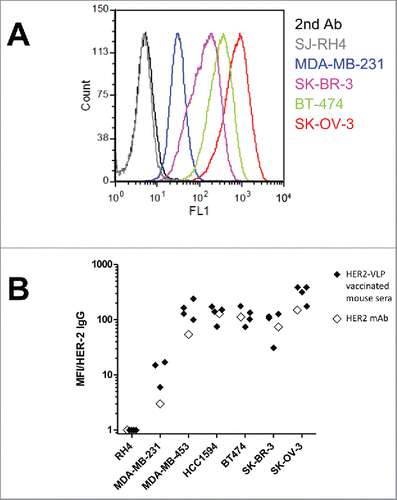
Figure 5. Subtypes, avidity and affinity of anti-HER2 IgG Abs elicited by vaccination with HER2-VLP. (A) IgG subtypes elicited by the indicated vaccinations, determined by flow cytometry of SK-OV-3 cells after indirect immunofluorescence with subtype-specific secondary Abs. Each bar represents the mean and SEM of 2–5 individual sera of different mice obtained two weeks after the third vaccination. (B) Avidity index, as determined by avidity ELISA, of Abs elicited by the indicated vaccinations. Each point represents the mean±SEM of 3–5 sera. The slopes of linear regression lines are significantly different (p < 0.02). (C, D) The Attana Quartz Crystal Microbalance biosensor was used to measure dissociation rates for the kinetic binding between recombinant HER2 ECD and purified total IgG from VLP-HER2 immunized mice (C) or Trastuzumab mAb (D). Different dilutions of anti-HER2 IgG were flushed over a surface of recombinant HER2 ECD immobilized at 50 µg/mL. Binding is shown as change in frequency over time (ΔHz). The black curve represents the real-time trace, while the red curve shows the fit of the dissociation rate measured for 500 seconds (1000–1500 seconds after injection). Dissociation rate constants (Kd) were obtained by applying a dissociation rate model using the TraceDrawer software.
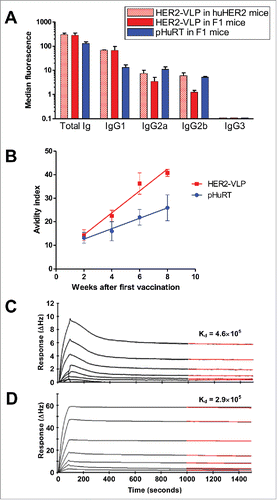
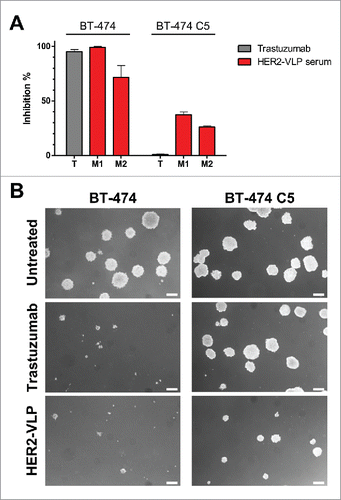
Figure 6. Antibodies elicited by the HER2-VLP vaccine inhibit 3D growth of human breast cancer cells. HER2-positive BT-474 cells and the trastuzumab-resistant clone BT-474 C5 were seeded in soft agar containing either trastuzumab (10 μg/ml) or sera (diluted 1:100) from two Delta16 mice (labelled as M1 and M2), vaccinated with HER2-VLP. The concentration of anti-HER2 Abs in mouse sera, as determined by ELISA, was comparable to that of trastuzumab (9.7–11.5 μg/ml). (A) Inhibition of agar colony number, counted 18 days after seeding. Each bar represents the mean (and SEM) inhibition of colony number in two independent plates. Inhibition by trastuzumab or anti-HER2 sera was calculated in reference to wells containing medium alone and normal mouse serum, respectively. (B) Dark-field micrographs of colonies in agar. Pictures labelled ‘HER2-VLP’ correspond to serum M1 in panel A. White bar corresponds to 200 μm.