Figures & data
Figure 1. Efficacy of GNP-LLO91-99 therapy on melanoma regression, metastases and mouse survival. (a) Scheme of GNP-LLO91-99 therapy. B16OVA melanoma cells were auto-transplanted s.c into the right hind flanks of female C57BL/6 mice. Seven days later, the mice were inoculated i.v with a single dose of GNP-LLO91-99 (50 µg/mouse) nanotherapy. Seven days post-nanotherapy, the mice were examined, blood obtained, serum stored for evaluation of cytokine concentrations and the mice were then killed. Spleens were removed to measure general immune responses. Melanomas were homogenized, filtered and centrifuged in Ficoll gradients to isolate TILs in the interphase and melanoma (MEL) in pellets. (b) B16OVA melanoma auto-transplants established s.c (n = 10/group of mice, left plots) were inoculated i.v or not (NT) with a single dose of the following therapies: LLO91-99 or LLO189-201 peptides (50 µg/mouse), control GNP nanovaccines coated with glucose (50 µg/mouse), GNP-LLO91-99 (5 or 50 µg/mouse), GNP-GAPDH1–22 (50 µg/mL) or DC-LLO91-99 (106 cells/mouse). Melanomas were removed and measured with a calliper. Tumour volumes (mm3) are expressed as the mean ± SD. Right images correspond to B16OVA melanoma s.c allo-transplants established in P4 neonates of CD1 mice. After 4 days, the mice were treated in situ with 50 µg/mouse of the following therapies: GNP-LLO91-99, control GNP, LLO91-99 peptide, GNP-GAPDH1-22 or DC-LLO91-99 (106 cells/mouse). (c) The number of lung metastases in mice in B were quantified. The results are expressed as the mean lung metastases ± SD. (d) B16OVA melanoma was s.c auto-transplanted into the right hind flanks of C57BL/6 mice for 7, 14, 23 or 30 days (n = 10/group of mice) and i.v inoculated or not (NT) with a single dose of the following therapies: control GNP nanovaccines coated with glucose (50 µg/mouse), GNP-LLO91-99 (50 µg/mouse), DC-GNP-LLO91-99 (106 cells/mouse), DC-GNP-GAPDH1-22 (106 cells/mouse) or DC-LLO91-99 (106 cells/mouse). The number of surviving mice was counted on day 7, 14, 23 or 20 post-vaccination. Asterisk highlights that plots of DC-GNP-LLO91-99 and GNP-LLO91-99 mice groups, completely overlap. Survival rates (SR) are expressed as the mean ± SD (P ≤ 0.05). Mice survivors of GNP-LLO91-99 groups were separated and maintained for at least 3 months to check for any tumours, or clinical manifestations. Afterwards, mice were scarified and check internally for any tumour metastasis. All survivors remained healthy and without tumours, suggesting tumours cured.
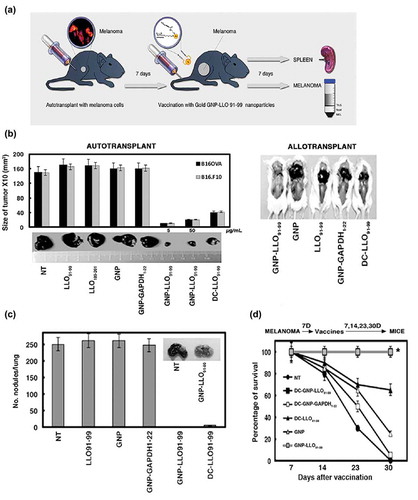
Figure 2. GNP-LLO91-99 nanotherapy affects melanoma programmed cell-death and antigen-presentation markers. (A, B) B16OVA melanoma auto-transplants were established s.c as in (n = 10/group of mice) and vaccinated i.v with control GNP (50 µg/mouse), GNP-LLO91-99 (50 µg/mouse) or non-treated (NT). After 7 days, histochemistry and immunohistochemistry were performed on melanoma embedded in paraffin to calculate the percentage of necrosis (A) or stained (B) with haematoxylin-eosin (HE). Asterisks over NT bars indicate that all groups of mice were compared to NT. The results are expressed as the mean number of necrotic foci ± SD (P ≤ 0.05). In (B), we show representative images of each group of mice stained with HE and mark with an asterisk, the NT group to which we compared all samples. (C) Removed melanoma were analysed for apoptosis by FACS analysis. We measured levels of early apoptosis using single staining with the apoptotic marker annexin-V (Annexin V) or late apoptosis using double staining with the DNA marker 7-AAD and the apoptotic marker annexin-V. Upper plots labelled as in vivo correspond to a representative experiment and the percentages reflect the cells localized in each quadrant from a total of 50.000 cells passed by the flow cytometer. GNP-LLO91-99 treated samples are compared to NT and significant differences are marked with an asterisk (*). The experiment was performed three times and results of each group of 10 mice are the following for late apoptosis (7-AAD+AnnV+ double positive cells): B16OVA/GNP-LLO91-99, 73 % ± 0.5; B16OVA/NT*, 50 % ± 1.5; B16.F10/GNP-LLO91-99, 76 % ± 1.5; B16.F10/NT*, 45 % ± 1.0. Results for early apoptosis (7-AAD−AnnV+) are: B16OVA/GNP-LLO91-99, 7 % ± 0.5; B16OVA/NT, 13 % ± 1.0; B16.F10/GNP-LLO91-99, 17 % ± 1.5; B16.F10/NT, 9 % ± 0.5 (P ≤ 0.05). We also performed in vitro experiments of different tumour cells (lower bar plot), melanoma (B16OVA, B16.F10, A-375), ovary CHO or macrophage IC-21 cell lines treated for 16 h with GNP-LLO91-99 (50 µg/ml) (black bars) or supernatants of DC cells pre-treated with GNP-LLO91-99 (50 µg/ml) (grey bars) and, next, analysed for early apoptosis by FACS. Results correspond to the mean of five different experiments and are expressed as the mean percentage of positive cells ± SD (P ≤ 0.05). (D) Analysis of antigen-presentation and cell-death markers on the cell surface of different melanoma cell lines, either murine B16OVA or human A375 as primary melanoma or murine B16.F10 or human MeWo as metastatic melanoma, were treated in vitro with GNP-LLO91-99 nanovaccines (50 µg/mL, 16 h, 37ºC) (+ GNP bars). Cells were stained with different fluorescent-labelled antibodies and examined by FACS analysis. The results are expressed as the mean percentage of positive cells ± SD (P ≤ 0.05).
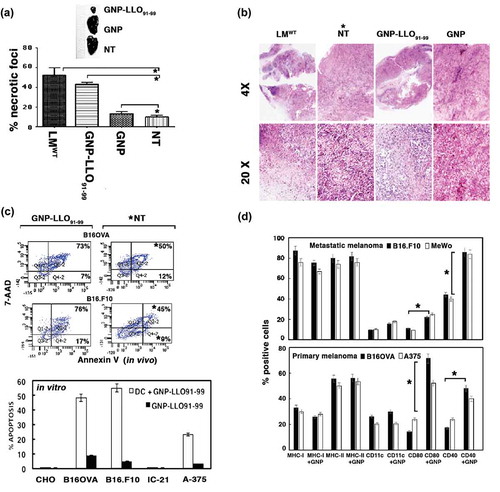
Table 1. Cytokine pattern in mice auto-transplanted with melanoma and treated with GNP-LLO91-99 nanovaccines.
Figure 3. GNP-LLO91-99 nanotherapy as an immune effector: analysis of TILs, spleens and cytokines in sera. (a) B16.F10 and B16OVA melanoma auto-transplants were established s.c as in - (n = 10/group of mice) and then i.v inoculated or not (NT) with a single dose of GNP-LLO91-99 (50 µg/mouse). After 7 days, melanomas were removed and TILs isolated. TILs were analysed by FACS and tumour infiltrated (Ti)-DCs with CD11c+CD8α+ phenotypes examined for different markers with specific monoclonal antibodies. Data in the plots correspond to B16.F10 melanoma and images correspond to melanoma embedded in paraffin and stained with anti-CD123 to stain DCs. We also quantified the amount of CD123+ cells in recovered melanoma by FACS, marked with an asterisk, and detected the following percentages: untreated B16.F10, 10% ± 0.9; GNP-LLO91-99 treated B16.F10, 87% ± 1.3; untreated B16OVA, 9% ± 0.6; GNP-LLO91-99 treated B16OVA, 84% ± 1.2. The results are expressed as percentages of positive cells ± SD (P ≤ 0.05). (b) Treg cell populations in spleens and melanoma were analysed by FACS. GNP-LLO91-99 treated samples are compared to NT samples and marked with an asterisk (*) to indicate significant decreases. These experiments have been performed five times. The summarizing data of percentages of CD4+FoxP3+ positive cells (Treg) are the following in the spleens: NT group: 16 % ± 2.0 and GNP-LLO91-99 group: 14.4 % ± 1.6. While Treg percentages in melanoma are: NT group: 18% ± 1.5 and GNP-LLO91-99 group: 3.6 % ± 0.4 (P ≤ 0.05). (c) CTL activities specific of each peptide were examined with the frequencies of LLO91-99 or OVA257-264 peptide-specific CD8+ cells and IFN-γ producers in TILs. We examined these specific CTL activities using recombinant soluble dimeric mouse H-2b: Ig fusion protein that bind to each peptide, as described in the Methods and materials section. The results are expressed as percentages of positive cells ± SD.
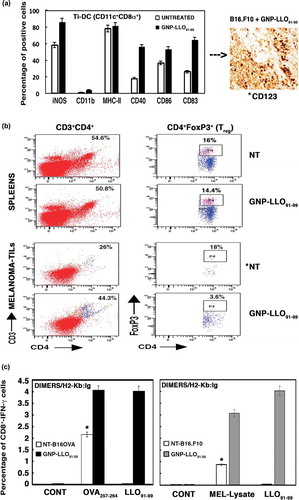
Figure 4. Pre-clinical studies with GNP-LLO91-99 nanovaccines as immunotherapies in combination with checkpoint inhibitors in mice and melanoma patients. (a) B16OVA melanoma auto-transplants established as in (n = 10/group of mice) were i.v vaccinated or not (saline) with a single dose of GNP-LLO91-99 (50 µg/mouse) alone or in combination with anti-CTLA-4 or anti-PD-1 (100 µg/mouse every two days). At 7, 14, 23 or 30 days post-transplantation, melanomas were removed and measured with a calliper. Tumour volumes were expressed as the mean of mm3 ± SD. (b) The number of surviving mice in (a) was counted at days 7, 14, 23 or 30 post-vaccination. GNP-LLO91-99 and anti-PD-1 + GNP-LLO91-99 results completely overlaped (mark with an asterisk *). Survival rates (SR) are expressed as the mean percentages ± SD. Mice survivors of GNP-LLO91-99 groups were separated and maintained for 20 months to check for any tumours, or clinical manifestations. Afterwards, mice were scarified and check internally for any tumour metastasis. All survivors remained healthy and without tumours, suggesting tumours cured. (c) CTL activities specific of each peptide were examined with the frequencies of LLO91-99 or OVA257-264 peptide-specific CD8+ cells and IFN-γ producers in TILs as in , in mice non-treated (NT), GNP-LLO91-99, anti-CTLA-4 or anti-PD-1 treated or GNP-LLO91-99 treated in combination either with anti-CTLA-4 or anti-PD-1. We examined these specific CTL activities using recombinant soluble dimeric mouse H-2b: Ig fusion protein that bind to each peptide, as described in the Methods and materials section. The results are expressed as percentages of positive cells ± SD. (d) Ex vivo differentiated MoDC from a stage IIIB melanoma patient with the following phenotype CD45+CD11c+CD14− were incubated or not (CONT) with GNP-LLO91-99 (50 µg/mL), nivolumab (100 µg/mL), ipilimumab (100 µg/mL) or a combination of GNP-LLO91-99 with nivolumab or ipilimumab for 48 h, at the same concentrations as the monotherapies. Antigen-presentation (MHC-I, MHC-II, CD80 and CD86) and cell-death (PD-1, PD-L1 and Annexin-V) surface markers were analysed by FACS using specific monoclonal antibodies. The results are expressed as the mean percentages of positive cells ± SD (P ≤ 0.05). (e) Different melanoma cell lines, either primary melanoma as A-375 or MelJuSo, or metastatic melanoma as MeWo or SK-Mel24 were incubated with 50 µg/ml of GNP-LLO91-99 or ½ supernatants of ex vivo differentiated MoDC from a stage IIIB melnanoma pre-treated for 16 hours with 50 µg/ml of GNP-LLO91-99. Next, early apoptosis was examined by FACS as the percentages of positive cells for annexin V. (f) Model of the action of GNP-LLO91-99 nanovaccines as immunotherapies. GNP-LLO91-99 stimulates the priming and effector arm of immune responses: (1) as immune stimulators, they activate intra-tumoural DCs, as well as DCs in other locations to release cytokines with anti-tumoural capacities such as IL-12 and TNF-α and (2) as immune effectors, they recruit and potentiate the antigen-presentation abilities of DCs to TILs, inducing the activation of antigen- and melanoma-specific CD8+ T cells causing tumour necrosis and diminishing the amount of Treg in TILs. Therefore, dormant specific anti-melanoma immune responses in TILs were stimulated.
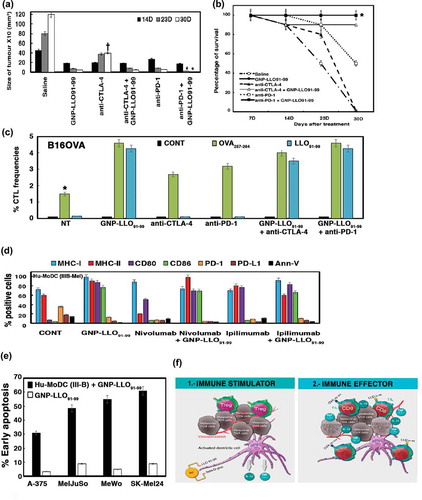
Table 2. GNP-LLO91-99 nanovaccines as human immunotherapies in comparison to check point inhibitors using MoDC.
