Figures & data
Figure 1. CD47 antibody retards the growth of spontaneous tumors. Medroxyprogesterone acetate (MPA) pellets (50 mg, 90-day release) were implanted subcutaneously into the interscapular area of immunocompetent C57BL/6 mice. Then the animals received 1 mg dimethylbenzantracene (DMBA) administered by oral gavage 6 x during 7 weeks. When spontaneous tumors became palpable, primary tumors (PT) were either excised and used for the flowcytometric detection of CD47 surface expression (a) or mice were randomly assigned to receive 10 mg/kg anti-CD47 antibody or isotype control that was administered intraperitoneally (i.p.). The antibodies were administered 4 times a week and then discontinued (Schedule I, b,d,f) or continuously given throughout the experiment (Schedule II, c,e,g). The tumor area was measured using a caliper until ethical endpoints were reached and animals had to be sacrificed (d,e). Experiments were performed with 12 animals per group and isotype controls from schedules I and II were pooled for tumor growth and survival analyses. Data were analyzed with TumGrowth (https://github.com/kroemerlab) to calculate significances and overall survival (f,g). The spleen from representative tumor-bearing C57BL/6 that were treated with the indicated antibodies were resected at the end of the experiment and photos were taken to document the increase in organ size (h,i).
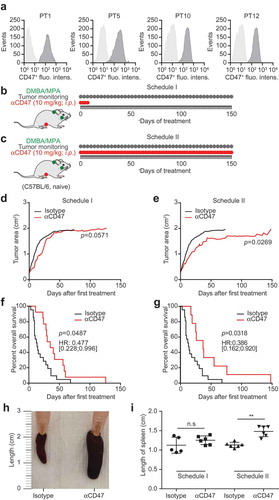
Figure 2. CD47 antibody decreases the growth of transplanted tumors. CD47 surface expression was detected in murine breast cancer 4T1 and AT3 cells by flow cytometry (a). One million murine breast cancer AT3 cells were inoculated subcutaneously into immunocompetent syngeneic C57BL/6 mice. Palpable tumors were injected intraperitoneally (i.p.) with 10 mg/kg anti-CD47 antibody or isotype control. Antibody or isotype control was administered 4 times a week for one week and then discontinued (Schedule I, b,d,f) or continuously (5 times a week) injected until the end of the experiment (Schedule II, c,e,g). The tumor area was measured using a caliper until ethical endpoints were reached when animals had to be sacrificed. Mean values of 8 animal per group are depicted (d,e). Data were analyzed with TumGrowth (https://github.com/kroemerlab) to calculate statistical differences and overall survival (f,g). The spleens from representative tumor-bearing C57BL/6 that were treated with the indicated antibodies were resected at the end of the experiment, and photos of the organs were taken to document morphological changes (h,i).
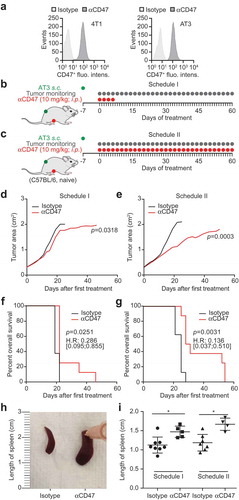
Figure 3. CD47 antibody-mediated tumor growth suppression is T cell-dependent. One million murine breast cancer AT3 cells were inoculated subcutaneously in immunocompetent syngeneic C57BL/6 mice. Palpable tumors were treated with 10 mg/kg anti-CD47 antibody or isotype control administered intraperitoneally (i.p.) together with or without mitoxantrone (MTX)-based chemotherapy (5.17 mg/kg). Antibody or isotype control was administered 5 times a week, while chemotherapy was administered once only. For T cell depletion, CD4 and CD8-specific antibodies (0.4 mg/mouse each) were injected together with chemotherapy and then once per week (a). The tumor area was measured using a caliper until ethical endpoints were reached. Tumor growth curves are individually depicted (b–e) and summarized as mean values (f). Data were analyzed with TumGrowth (https://github.com/kroemerlab) to calculate significances and overall survival (G).
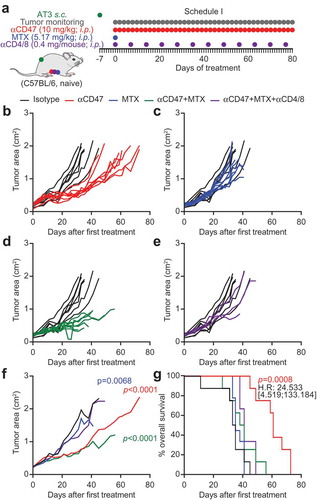
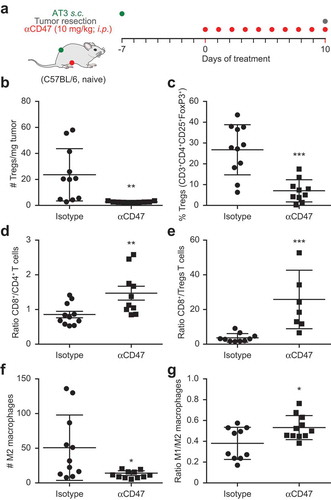
Figure 4. Immunotherapy with CD47 antibody induces changes in tumor immune infiltrate. Mouse breast cancer AT3 tumors were established subcutaneously and mice received 10 mg/kg anti CD47 antibody daily (a). At day 10 tumors were excised and dissociated followed by staining with exclusion dye and the immune detection of surface markers and intranuclear Foxp3 by flow cytometry. Data was analyzed by GraphPad and subjected to outlier exclusion by means of the Grubbs’ test. The absolute number of regulatory T cells (Tregs) per tumor mass and the percent of Tregs decreased in response to CD47 antibody immunotherapy (b,c), whereas the ratio of CD8+ over CD4+ and ratio of CD8+ over Tregs increased (d,e). The number of M2 macrophages decreased and the ratio of M1 over M2 macrophages increased (f,g). Results were subjected to unpaired Student t tests (*p < 0.05; **p < 0.01; ***p < 0.001).