Figures & data
Figure 1. E7 RNA-LPX immunization induces a strong antigen-specific effector and memory CD8+ T cell response in mice (a-d) Mice (n=5/group) were immunized twice with E7 RNA-LPX or NaCl (control) (d0, d7) and blood samples were harvested six days after each immunization (d6, d13). (a) Serum cytokines 3 h after first immunization were determined by multiplex immunoassay. (b-d) Flow cytometric characterization of de novo primed E749-57 MHC-class I multimer positive CD8+ T cells in the blood with regard to (b) their frequency and (c, d) their phenotype on d13. (c) Differential expression of CD127 and KLRG1 on E749-57 MHC-class I multimer positive (pos) and negative (neg) CD8+ T cells of E7 RNA-LPX immunized mice. (d) Differential expression of CD62L within KLRG1−CD127+ (MPEC) E749-57-specific CD8+ T cells. (b, c) Representative pseudocolor plots are shown. Significance was determined using (a, b) unpaired, two-tailed Student’s t-test and (c) two-way ANOVA, Bonferroni post-test. Mean±SD. SLEC: Short-lived effector cells; MPEC: Memory precursor effector cells; Tem: T effector memory cells; Tcm: T central memory cells.
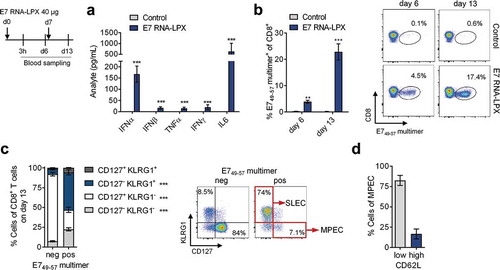
Figure 2. E7 RNA-LPX immunization mediates complete remission of progressing HPV16-positive tumors and establishes protective T cell memory (a-d) TC-1 luc tumor cells were grafted into the submucosal lining of the tongue. Five days later mice were immunized with E7 RNA-LPX (n=13) or irrelevant (OVA257-264) RNA-LPX (n=12). (a) TC-1 luc tumor growth kinetics as measured by in vivo bioluminescence. (b-d) Mice were sacrificed 10 days after tumor-challenge and tumor tissues harvested (n=3/group). The proportion of (b) CD45+ and (c) E749-57 multimer+ CD8+ T cells in mice treated either with E7 RNA-LPX or irrelevant (OVA257-264) RNA-LPX was measured by flow cytometry. (d) CD49α expression of E749-57 multimer+ CD8+ T cells of E7 RNA-LPX treated mice in blood and tumor. (e, f) TC-1 tumor growth (e, left) and survival (e, right) in mice (n=10/group) immunized three times with E7 RNA-LPX or irrelevant (eGFP) RNA-LPX. Average tumor size of ~6 mm3 at start of treatment. (f) Survival of E7 RNA-LPX treated mice (n=10) rechallenged with TC-1 after initial TC-1 tumor challenge. Treatment-naive mice (n=10) served as control group. (g, h) C3 tumor growth (g, left) and survival (g, right) in mice immunized four times with E7 RNA-LPX (n=9) or irrelevant (OVA257-264) RNA-LPX (n=10). Average tumor size of ~25 mm3 at start of treatment. (h) Survival of E7 RNA-LPX treated mice rechallenged with TC-1 after C3 tumor challenge (n=12/group). Treatment-naive mice (n=10) served as control group. (i, j) Mice (n=10) bearing C3 tumors (average size of 25 mm3 at start of treatment) were immunized four times with E7 RNA-LPX and left without further treatment after complete tumor rejection. (i) CD8+ T cell phenotype in blood was analyzed before (pre-boost) and 5 days after receiving a booster dose (post-boost) of E7 RNA-LPX or NaCl (n=5/group). (j) Specific lysis of target cells in vivo was determined 18 h after adoptive transfer of CFSE-labeled splenocytes pulsed with E749-57 or OVA257-264 (control) peptide. Significance was determined using (e-h) log-rank test, (a, i) two-way ANOVA, Sidak’s multiple comparison test, and (j) one-way ANOVA, Tukey’s multiple comparison test. Ratios depict the frequency of mice with complete tumor responses (e, g). Mean±SD.
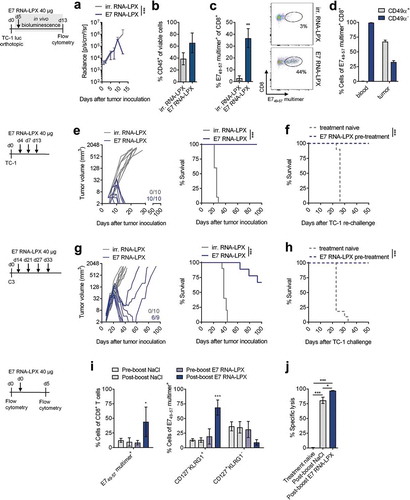
Figure 3. Tumors of E7 RNA-LPX immunized mice have brisk immune infiltrates, E7-specific CD8+ T cells and a proinflammatory, cytotoxic and less immune-suppressive contexture Analysis of TIL in TC-1 (a, b, c-e) and C3 tumors (a, b, f-h) resected from mice (n=5/group) 11 days after one immunization with E7 RNA-LPX or irrelevant (OVA257-264) RNA-LPX (TC-1: ~63 mm3 and C3: ~65 mm3 at therapy start). (a) Percentage of CD45+ cells and (b) frequency of E749-57-specific CD8+ T cells in TC-1 and C3 tumors as measured by flow cytometry. (b, right) Representative E749-57 multimer staining in TC-1 tumors. (c, f) Frequency of leukocyte subsets in (c) TC-1 and (f) C3 tumors. (d, g) Ki67+ fraction of tumor-infiltrating CD4+ and CD8+ T cells in (d) TC-1 and (g) C3 tumors. (e, h) Expression of iNOS and CD206 in TAM of (e) TC-1 and (h) C3 tumors. (i) Immunofluorescent staining of CD8+ T cell infiltration (green) in TC-1 (top) and C3 (bottom) tumors (nuclear staining: Hoechst (blue), scale bar = 50 µm). (j) Gene expression analysis of selected genes in TC-1 (top) and C3 tumors (bottom) resected after two immunizations with E7 RNA-LPX or irrelevant (OVA257-264) RNA-LPX determined by qRT-PCR. Tumor tissues were harvested two days after the last immunization. Heatmaps display log2-fold changes calculated and normalized to irrelevant (OVA257-264) RNA-LPX treated mice. (a-h) Significance was determined using unpaired, two-tailed Student’s t-test and (j) Holm-Šídák multiple comparison test. Mean±SD. TIL: Tumor-infiltrating lymphocytes; TAM: Tumor-associated macrophages; iNOS: Inducible nitric oxide synthase.
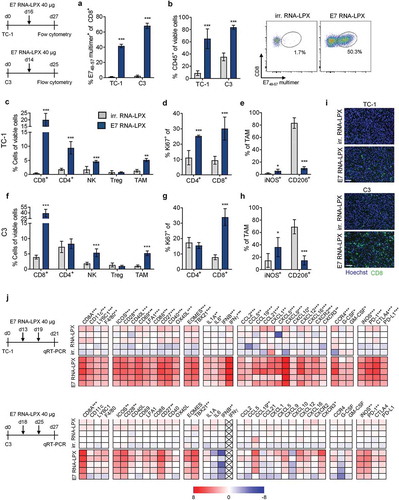
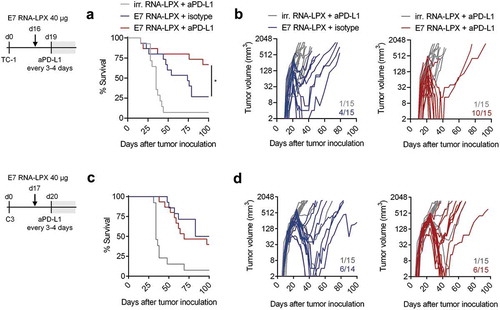
Figure 4. E7 RNA-LPX immunization synergizes with checkpoint-blockade by rendering anti-PD-L1 refractory tumors responsive (a, c) Survival and (b, d) tumor growth kinetics of (a, b) TC-1 or (c, d) C3-bearing mice (n=14–15/group) immunized with E7 RNA-LPX or irrelevant (OVA257-264) RNA-LPX on day 16 or day 17 respectively and treated with anti-PD-L1 (aPD-L1) antibody or isotype control from day 19 or day 20 onwards every 3 to 4 days. The average size of TC-1 tumors was ~48 mm3 and of C3 tumors at ~157 mm3 at start of treatment. Representative data is shown for two (each TC-1 and C3) similar, but independent experiments. Significance was determined using (a, c) log-rank test. (b, d) Ratios depict frequency of mice with complete tumor responses.