Figures & data
Figure 1. Anti-ErB2 and anti-PD-L1 mAb therapy in rat ErbB2-positive mouse tumors. (a) Her2 expression and PD-L1 upregulation on TUBO cells upon IFN-γ stimulation. TUBO cells were stimulated with recombinant mouse IFN-γ for 72 h. PD-L1 and Her-2 expression were quantified on the stimulated cells at 24, 48 and 72 h using a QIFI kit and MPDL3280A and 7.16.4 antibodies, respectively. H2N100 (b, c) and TUBO (d, e) tumor cells (5 × 105 cells) were injected subcutaneously into Balb/c wild type mice, and treated with 10 μg or 50 μg of anti-ErbB2 mAb (7.16.4), 200 μg anti-PD-L1 mAb or control Ig (200 μg) injected intraperitoneally on days 17, 21, 24 and 28. Mice were monitored for tumor growth and results are expressed as mean tumor area ± SEM. (c, e) Data is shown for the percentage of mice rejecting tumors/group (gray bars, tumor-bearing mice; black bars, tumor-free mice for at least 60 d). Arrow represents the day treatment was started. Statistical analysis was performed using two-way ANOVA Turkey’s multiple comparisons test at day 30 for B and day 39 for D (***, P < 0.0005; ****, P < 0.0001; ns: not significant).
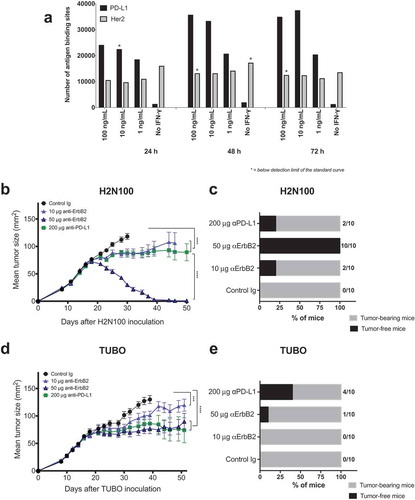
Figure 2. Binding of monospecific and bispecific antibodies to TUBO cells. TUBO tumor were cultured in the absence (a, b) or presence (c, d) of 10ng/ml of mouse IFN-γ for 24 h and incubated with different concentrations of FITC-labeled bivalent (homodimer) anti-rErbB2, anti-PD-L1, monovalent anti-rErbB2 (B12xErbB2) or anti-PD-L1 (B12xPD-L1) or bispecific BsPD-L1xrErbB2 antibodies. On the y-axes, the median fluorescence intensity (MFI) FITC is plotted against the antibody concentration in log nM on the x-axes. EC50 in nM for untreated TUBO (B) and IFN-γ treated TUBO (d) tumor cells is shown.
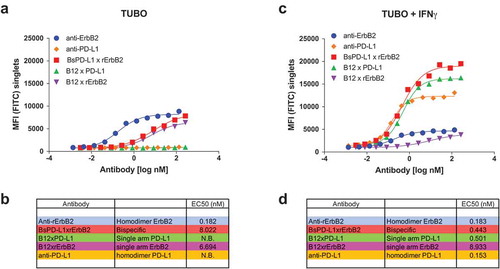
Figure 3. BsPD-L1xrErbB2 blocks PD-1/PD-L1 interaction but does not affect the viability of tumor cells in-vitro. TUBO tumor cells were cultured in the absence (a) or presence (b) of 10ng/ml of mouse IFN-γ overnight and cells were incubated with different concentrations of control Ig, anti-rErbB2, anti-PD-L1, monovalent anti-rErbB2 (B12xErbB2) or BsPD-L1xrErbB2 for 72 h. Viability of TUBO tumor cells is shown as determined by using the CellTiter-Glo system. (c) NFAT Reporter Activity (RLU) as a measure of PD-1/PD-L1 interaction are plotted on the y-axes against the antibody concentrations in log nM on the x-axes.
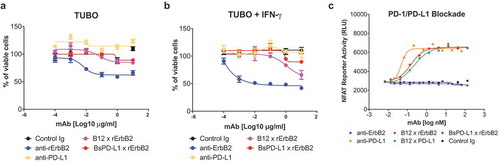
Figure 4. Treatment with BsPD-L1xrErbB2 bispecific antibody reduces tumor growth and increase tumor rejections. TUBO tumor cells (5 × 105 cells) were injected subcutaneously into Balb/c wild type mice, and treated with different concentrations of control Ig, anti-ErbB2 mAb (7.16.4), anti-PD-L1 mAb or BsPD-L1xrErbB2 bispecific antibody injected intraperitoneally on days 19, 22, 26 and 29 (a, b) or on days 16, 19, 23 and 26 (c, d). Mice were monitored for tumor growth and results are expressed as mean tumor area ± SEM. (b, d) Data is shown for the percentage of mice rejecting tumors/group (gray bars, tumor-bearing mice; black bars, tumor-free mice for at least 60 d). Arrow represents the day treatment was started. Statistical analysis was performed using two-way ANOVA Turkey’s multiple comparisons test at day 44 for A and day 28 for C (*, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.0001; ns: not significant).
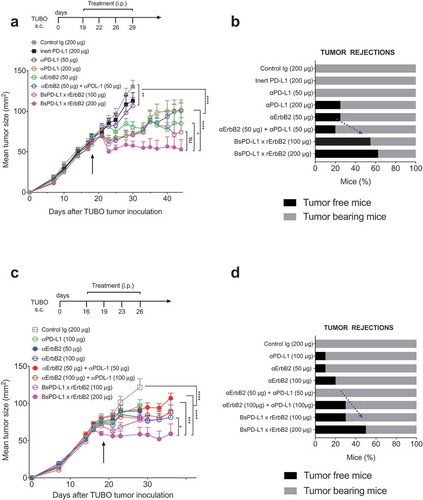
Figure 5. TUBO re-challenge in mice rejected tumors after bispecific antibody therapy. (a, b) Balb/c mice that rejected TUBO tumors after BsPD-L1xrErbB2, anti-ErbB2 alone, anti-PD-L1 alone or the combination therapy from different experiments were collected and 2 × 106 TUBO tumor cells were injected in the opposite flank of mice in each group and tumor growth was monitored. TUBO tumors did not grow in any of the group of mice that had rejected tumors before. (c) Data is shown for the percentage of mice rejecting tumors/group (gray bars, tumor-bearing mice; black bars, tumor-free mice for at least 60 d). Number represents the tumor-free mice/total number of mice in a group.
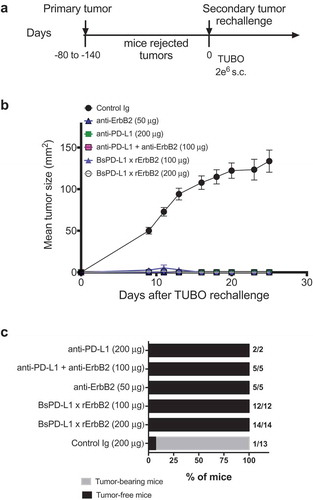
Figure 6. Increased CD8+ T cells in the tumor after BsPD-L1xrErbB2 bispecific antibody therapy. TUBO tumor-bearing mice were treated with two doses of cIg, anti-ErbB2 (7.16.4, 50μg), anti-PD-L1 (MPDL3280A, 50μg), the combination of anti-PD-L1 and anti-ErbB2 (called combination) or the BsPD-L1xrErbB2 at day 15 and 18, and 72 h after the last treatment tumors were resected and analyzed for the presence of TILs via flow cytometry. Percentage (a, c, e, g) and absolute number (b, d, f, h) of CD4+ T cells, CD4+FoxP3+ regulatory T cells (Tregs), CD8+ T cells and CD11b+Ly6Ghi granulocytic MDSC is shown. Data from individual mice are depicted by symbols in bar graphs. Results are expressed in mean ± SEM. Statistical analysis was performed using one-way ANOVA Turkey’s multiple comparisons test; *, P < .05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.0001 with n = 5–7 mice per group.
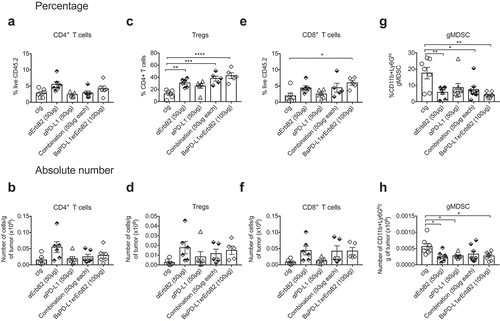
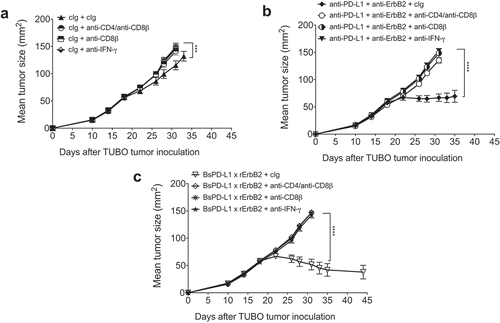
Figure 7. Mechanism of BsPD-L1xrErbB2 antibody therapy is dependent on CD8+ T cells and IFN-γ. TUBO tumor cells (5 × 105 cells) were injected subcutaneously into Balb/c wild type mice, and treated with (a) control Ig (b12, 200μg), (b) anti-PD-L1+ anti-ErbB2 combination (100μg each) or (c) BsPD-L1xrErbB2 bispecific (100μg) injected intraperitoneally on days 19, 22, 26 and 29. Additionally, some groups of mice were treated with control IgG (cIg), CD4- and CD8-depleting anti-CD4/anti-CD8β antibodies or IFN-γ neutralizing anti-IFN-γ antibodies. Mice were monitored for tumor growth and results are expressed as mean tumor area ± SEM. Statistical analysis was performed using two-way ANOVA Turkey’s multiple comparisons test (***, P < 0.0005; ****, P < 0.0001).