Figures & data
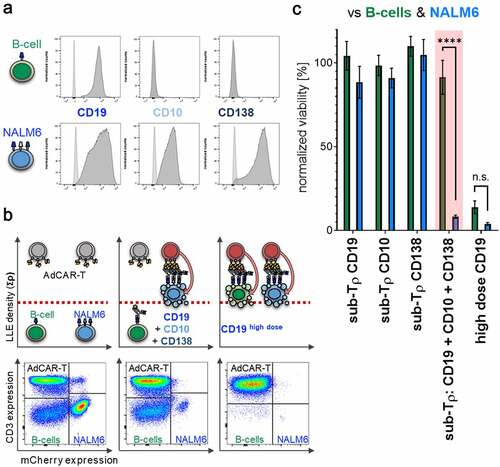
Figure 1. Design and characterization of the AdCAR-T system
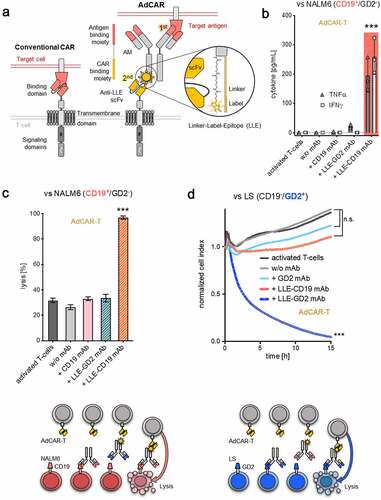
Figure 2. Specificity and sensitivity of the AdCAR-T system
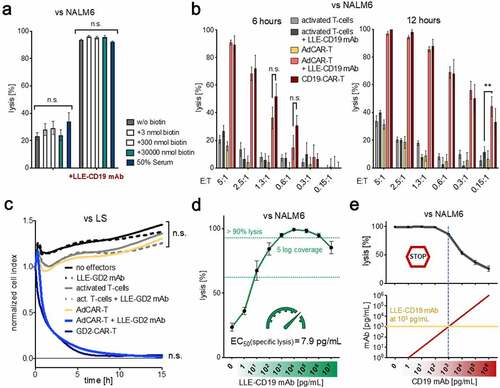
Figure 3. In vivo evaluation of AdCAR-T
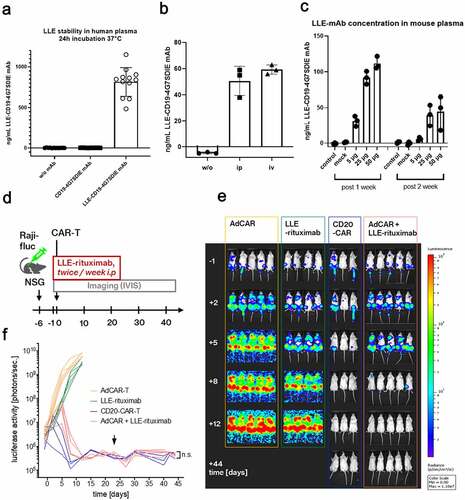
Figure 4. Universal targeting and combinatorial targeting to avoid antigen evasion
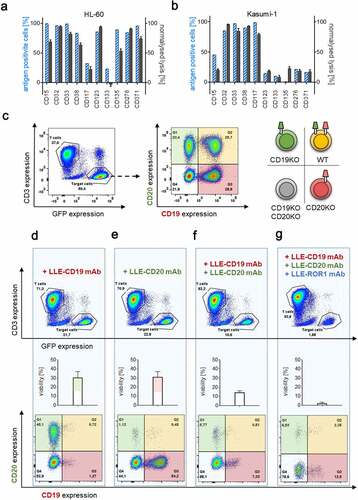
Figure 5. Sequential combinatorial targeting mediated by AdCAR-T
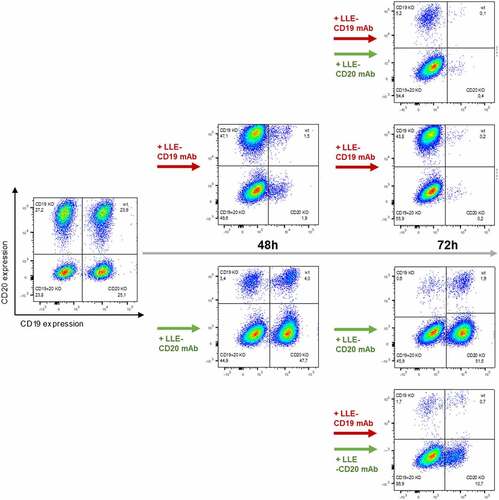
Figure 6. Surface Activation Matrix (SAM) a new concept of targeted polyimmunotherapy
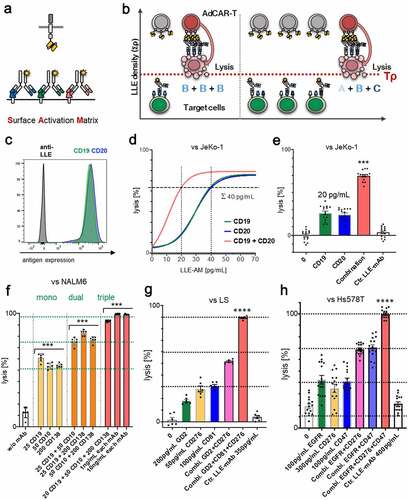
Figure 7. Identification and target cell lysis by integration of antigen expression profiles in AML
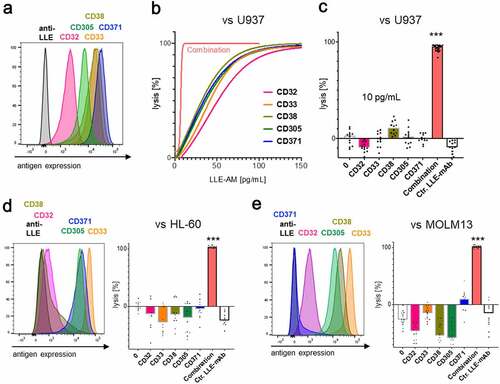
Figure 8. Differential target cell lysis by AdCAR-T