Figures & data
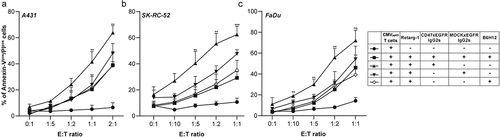
Figure 1. Cancer cells under T cell immune attack upregulate cell surface CD47
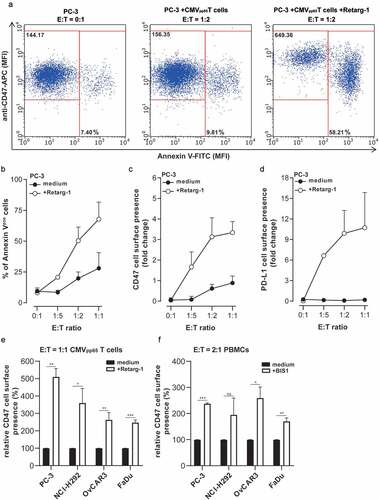
Figure 2. Upregulation of CD47 expression enhances resistance of cancer cells to T cell-mediated cytotoxicity
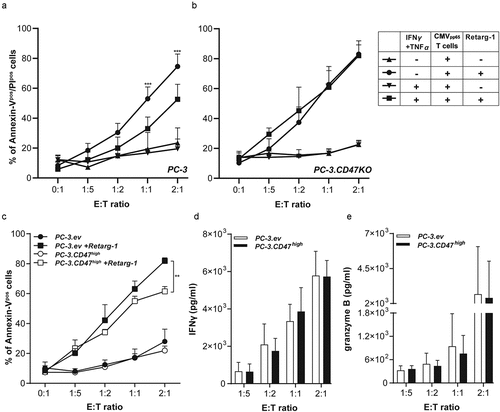
Figure 3. CD47 knockout enhances susceptibility of cancer cells to T cell-mediated cytotoxicity
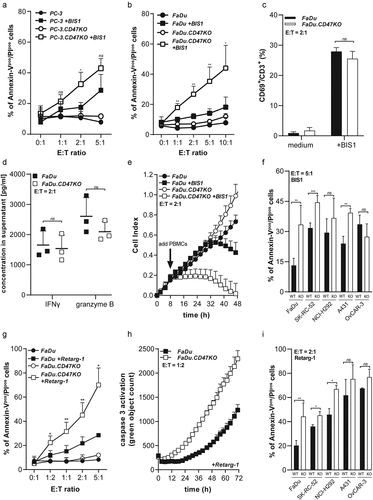
Figure 4. CD47 knockout enhances susceptibility of cancer cells to TRAIL- and FAS-mediated cell death
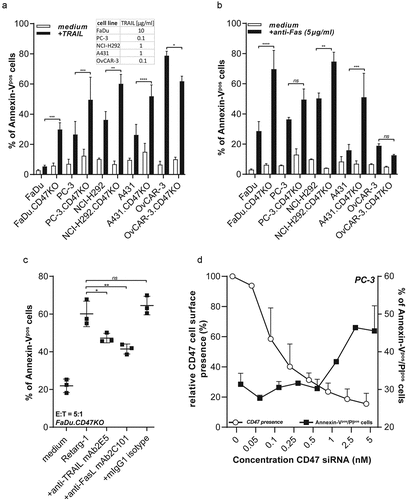
Figure 5. BsAb CD47xEGFR-IgG2s-mediated internalization of CD47 enhances susceptibility of cancer cells to TRAIL-mediated cell death
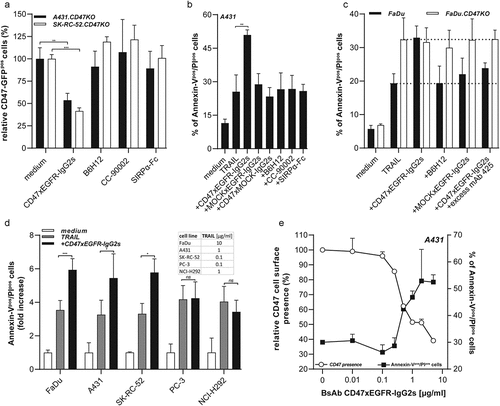
Figure 6. BsAb CD47xEGFR-IgG2s-mediated internalization of CD47 enhances susceptibility of cancer cells to T cell-induced cytotoxicity