Figures & data
Figure 1. Best percentage change from baseline and best overall responses in all patients (naive to prior anti-PD-1/L1 and pretreated) with mesothelioma, NSCLC, melanoma, RCC, and TNBC.
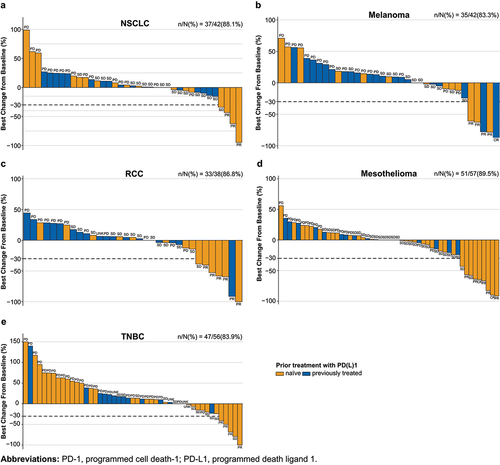
Figure 2. Duration of response per RECIST v1.1 in patients naive to prior anti-PD-1/L1 and pretreated patients.
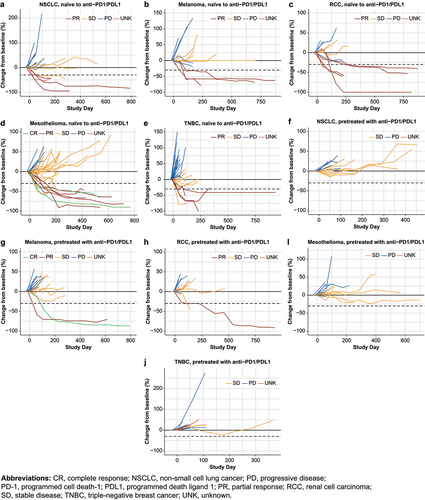
Table 1. Summary of best overall response, disease control rate, and progression-free survival by RECIST v1.1.