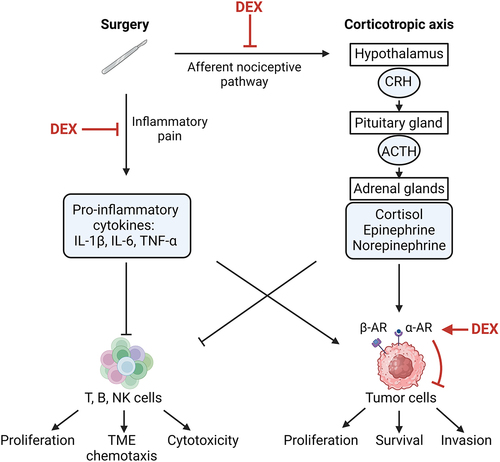
Figures & data
Figure 1. Dexmedetomidine: chemical formula and mode of action.
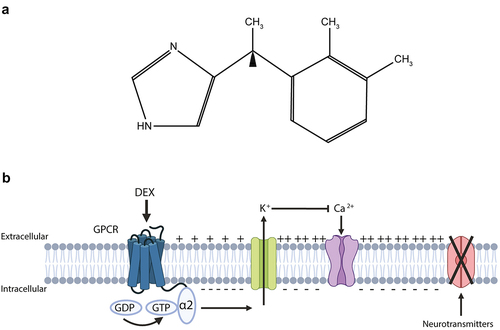
Figure 2. Sympatholytic and analgesic effects of dexmedetomidine through the α2-adrenoceptors.
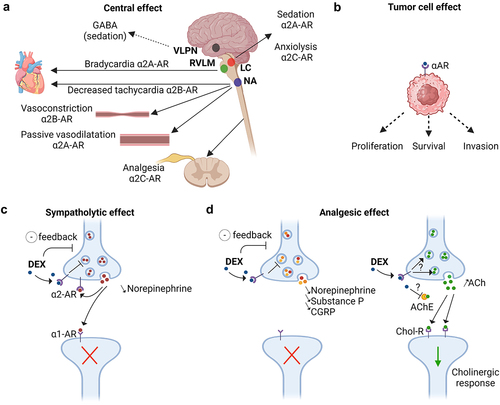
Table 1. Preclinical research describing protumor effects of dexmedetomidine.
Figure 3. Molecular mechanisms of dexmedetomidine-mediated anti-tumor effects.
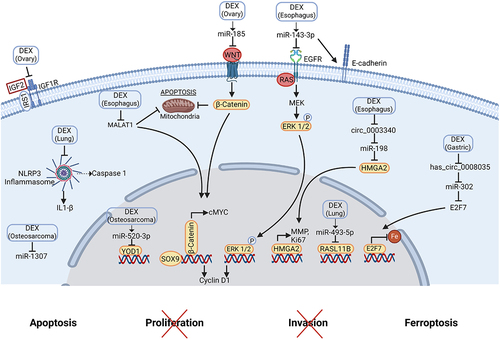
Table 2. Preclinical research describing antitumor effects of dexmedetomidine.
Table 3. Published clinical trials studying the dexmedetomidine-induced biological effects potentially related to oncological outcome.
Table 4. Completed trials investigating the role of dexmedetomidine on cancer outcome.
Table 5. Ongoing trials investigating the role of dexmedetomidine on cancer outcome.
Table 6. Completed and ongoing trials investigating the role of OFA on cancer outcome.
Figure 4. Scheme of central and peripheral actions of dexmedetomidine.