Figures & data
Figure 1. Structural and functional characterization of the PD-L1×TGF-β bispecific antibody. (a) Genetic structure of the tandem scFv anti-PD-L1 × anti-TGF-β named AxF (scFv)2. Oncostatin M signal peptide is used to direct the secretion of the recombinant antibody, and the myc/6×His tags (light blue and dark red boxes) were appended for immunodetection and affinity purification, respectively. A 3D model of AxF (scFv)2 was built with AlphaFold and refined using Pymol. (b) Reducing SDS-PAGE of the construct, (c) size exclusion chromatography analysis with the indicated molecular weight measured at the center of the chromatography peak (red line) and (d) circular dichroism analysis of the purified AxF (scFv)2. (e) ELISA titration against plastic-immobilized human PD-L1 or TGF-β of the purified AxF (scFv)2 and (f) dose-dependent binding curve to the CHOPD-L1 cell surface by FACS. (g) Simultaneous binding of the recombinant antibody to PD-L1 and TGF-β by two-step ELISA. (H) Serum stability of the AxF (scFv)2 incubated in mouse or human serum for 96 hours.
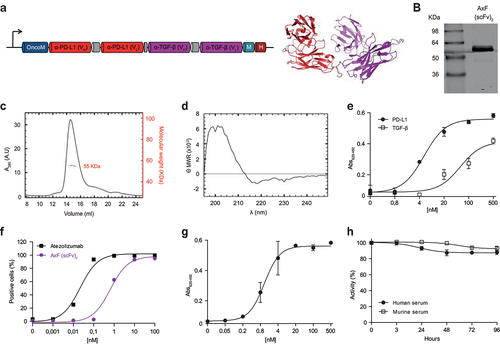
Figure 2. Disruption of PD-L1 and TGF-β signaling axes promoted by purified PD-L1×TGF-β bispecific antibody. (a) Dose-dependent blockade of TGF-β/TGF-βR interaction triggered by the AxF (scFv)2 using a mink lung epithelial cell (MLEC)-based reporter system. 1D11 IgG was used as a control. (b) Suppression of TGF-β-dependent gene expression in human pericytes treated with TGF-β in the presence of AxF (scFv)2, as assessed by qRT-PCR. (c) TGF-β-mediated increase of PDCD1 gene expression in T cells is reversed by AxF (scFv)2 and anti-TGF-β 1D11 IgG. Experiments were performed at least twice in triplicates. (d) Modulation of E- and N-cadherin expression in A549 cells treated with TGF-β in the presence of the AxF (scFv)2. (e) Inhibition of PD-1/PD-L1 axis by the AxF (scFv)2 as assessed with a luciferase-based reporter bioassay. Statistical differences were examined by unpaired Student’s t-test assuming a normal distribution. Mean ± SD are shown at each condition.
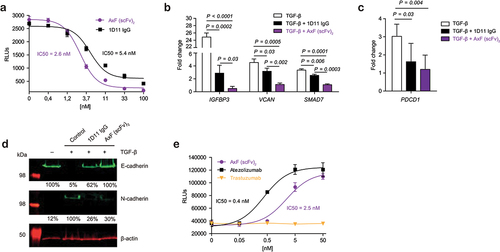
Figure 3. TriTE-mediated T cell activation, cytotoxicity and IFN-γ secretion is enhanced by purified PD-L1×TGF-β bispecific antibody. A549, HCT116 or SKBR3 cells were cocultured in 96-well plates with PBMCs at the effector/target (E:T) ratio of 5:1 in the presence of different concentrations of purified TriTE antibodies and 100 nM of AxF (scFv)2. (a) Expression of T cell activation marker CD69 at 24 hours, determined by FACS analysis. (b) Specific cytolysis of tumor cells as assessed by bioluminescence. Percent specific lysis was calculated relative to an equal number of tumor cells cultured with PBMCs in the absence of purified antibodies. EC50 values are provided according to the color code. (c) IFN-γ production was determined in CM by ELISA. PBMCs were obtained from 3 different donors, and experiments were performed in triplicate. Results are expressed as a mean ± SD.
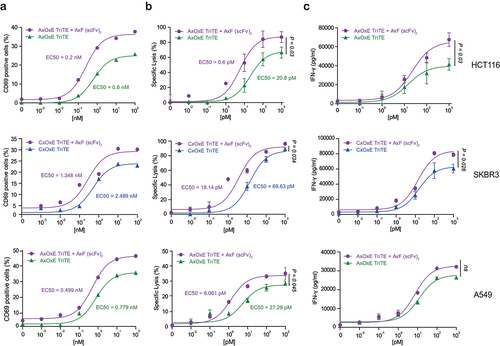
Figure 4. Cytotoxicity and IFN-γ secretion elicited by CAR-T cells improved by purified PD-L1×TGF-β bispecific antibody. HCT116 or SKBR3 cells were cocultured in 96-well plates CAR-EGFR or non-transduced (N-T) T cells at different E:T ratios in the presence of 100 nM of AxF (scFv)2. (a, b) After 48 hours, specific cytolysis of tumor cells were measured by bioluminescence assay. Percent specific lysis was calculated relative to an equal number of tumor cells cultured with N-T T cells in the absence of AxF (scFv)2. (c, d) IFN-γ secretion was determined in CM by ELISA. PBMCs were obtained from 3 different donors, and experiments were performed in triplicate. Results are expressed as a mean ± SD.
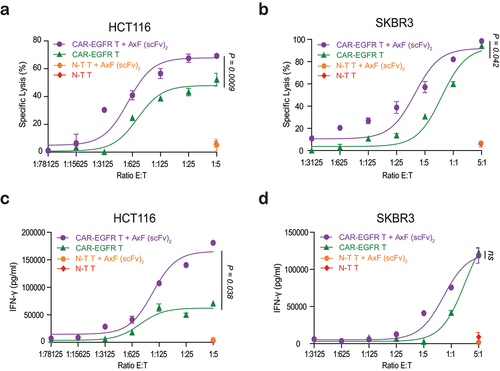
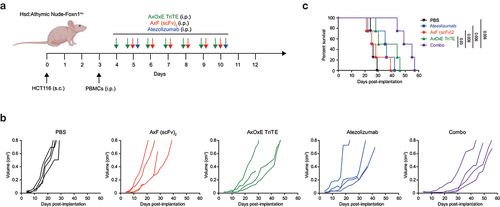
Figure 5. In vivo antitumor efficacy of EpCAMxCD3×EGFR TriTE enhanced by PD-L1×TGF-β bispecific antibody. (a) Hsd:Athymic Nude-Foxn1nu mice were inoculated subcutaneously with 2 × 106 HCT116 tumor cells. When tumors reached 0.3 cm in diameter, mice were randomized into groups (n = 4/group) and 107 freshly isolated PBMCs were injected intraperitoneally. PBS, atezolizumab, AxOxE TriTE and/or AxF (scFv)2 were administered intraperitoneally daily for 6 or 7 d. (b) Tumor volume growth curves for individual mice are represented for each group. (c) Kaplan-Meier survival curves for each treatment group are shown. Overall survival was analyzed with log-rank (Mantel-Cox) test.
Supplemental Material
Download Zip (4.8 MB)Data availability statement
The data that support the findings of this study are available from the corresponding author, [LS], upon reasonable request.