Figures & data
Table 1. The clinical characteristics of 40 patients (responder: n = 20, non-responder: n = 20) who received anti-PD-1 therapy.
Figure 1. The characterization of T cells with TCRs in the blood.
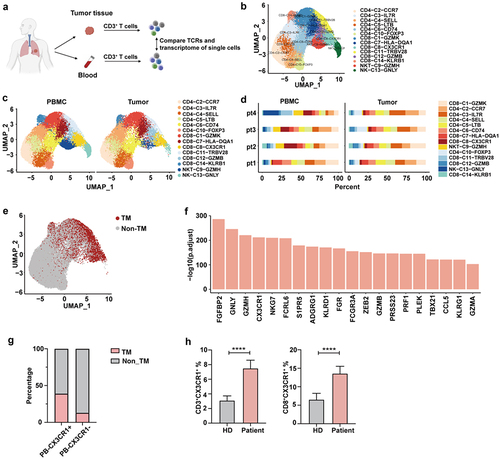
Figure 2. Characterization of tumor-matching T cells in peripheral blood.
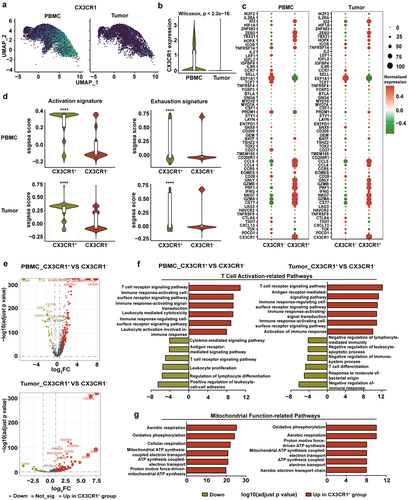
Figure 3. Peripheral CX3CR1+ T cells exhibit properties of activated effector cells.
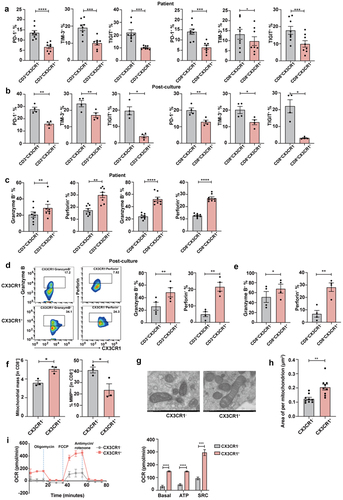
Figure 4. CX3CR1+ T cells display robust anti-tumor reactivity ability and decrease Fas-mediated apoptosis by anti-PD-1 antibody.
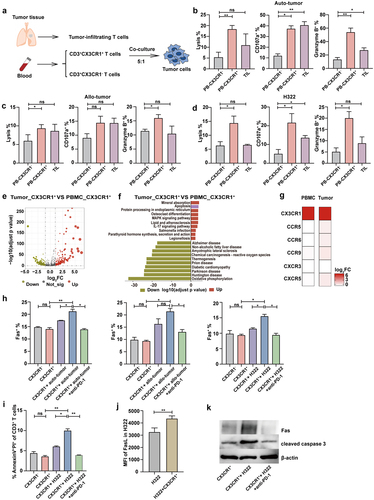
Figure 5. Combination therapy with CX3CR1+ T cells and anti-PD-1 therapy in lung cancer mouse model.
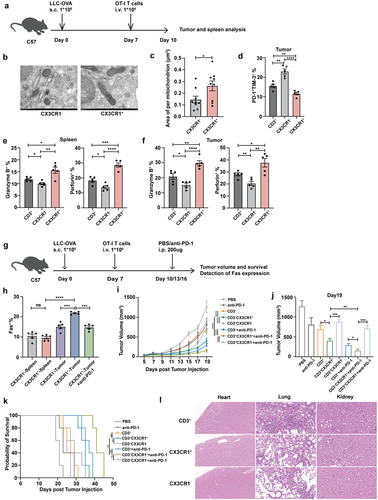
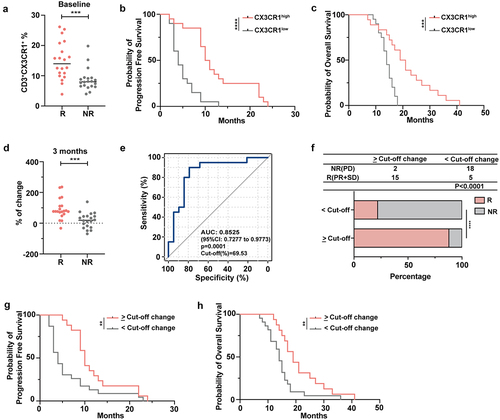
Figure 6. The frequency of circulating CX3CR1+ T cells correlates with effective ICI therapy.