Figures & data
Figure 1. β-Lap selectively kills NQO1+ murine tumor cells and induces extensive DNA damage. (a–b) NQO1 expression and activity in murine tumor cell lines. (a) Level of NQO1 protein expression analyzed by western blot. (b) NQO1 enzyme activity. (c–d) Viability of murine tumor cell lines expressing endogenous NQO1; 4T1 (c) and EO771 (d) following β-Lap with or without DIC (Dicumarol, an NQO1 specific inhibitor) treatment for 3 h determined by DNA assay. (e–f) ROS measurement in murine tumor cells. (g) γH2AX foci/nuclei immunofluorescence staining indicating DNA damage in the form of double strand breaks induced by β-Lap treatment. Scale bar = 10 μm (h–i) Quantification of γH2AX foci/nuclei. Data are shown as mean ± SD from three independent experiments. ***p < 0.001, ****p < 0.0001, ns: not significant (determined by unpaired Student’s t-test).
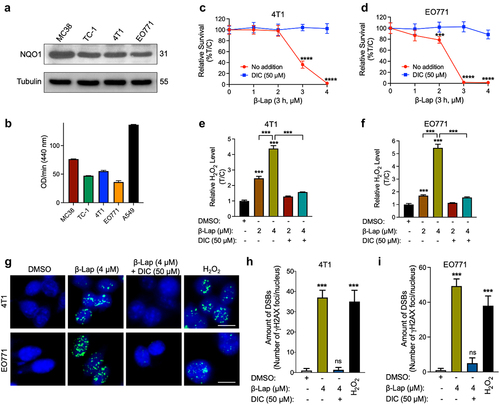
Figure 2. Impact of β-Lap treatment on tumor-infiltrating neutrophils in the tumor microenvironment. C57BL/6 WT mice (n = 5/group) were inoculated subcutaneously (s.c.) with 6 × 105 MC38, 1 × 106 EO771, or 1 × 105 TC-1 cells, while BALB/c WT mice (n = 5/group) received subcutaneous inoculations of 6 x 105 4T1 cells. Tumor-bearing mice were subsequently treated with β-Lap (25 mg/kg, i.v., for MC38 and TC-1, or 18 mg/kg, i.t. For 4T1 and EO771 tumors) or 20% HPβCD (Vehicle) every other day for a total of four treatment once tumor volumes reached ~80 mm3. Tumor samples were harvested 24 h after the last administrated dose. Tumor-infiltrating neutrophils were assessed in (a) MC38, (b) TC-1 [gated as CD11b+Gr-1high population], (c) 4T1 and (d) EO771 (gated with specific markers, as Ly6C−Ly6G+ population) tumors using flow cytometry. Data are presented as mean ± SD from three independent experiments. *p < 0.05, **p < 0.01 determined by unpaired Student’s t-test.
![Figure 2. Impact of β-Lap treatment on tumor-infiltrating neutrophils in the tumor microenvironment. C57BL/6 WT mice (n = 5/group) were inoculated subcutaneously (s.c.) with 6 × 105 MC38, 1 × 106 EO771, or 1 × 105 TC-1 cells, while BALB/c WT mice (n = 5/group) received subcutaneous inoculations of 6 x 105 4T1 cells. Tumor-bearing mice were subsequently treated with β-Lap (25 mg/kg, i.v., for MC38 and TC-1, or 18 mg/kg, i.t. For 4T1 and EO771 tumors) or 20% HPβCD (Vehicle) every other day for a total of four treatment once tumor volumes reached ~80 mm3. Tumor samples were harvested 24 h after the last administrated dose. Tumor-infiltrating neutrophils were assessed in (a) MC38, (b) TC-1 [gated as CD11b+Gr-1high population], (c) 4T1 and (d) EO771 (gated with specific markers, as Ly6C−Ly6G+ population) tumors using flow cytometry. Data are presented as mean ± SD from three independent experiments. *p < 0.05, **p < 0.01 determined by unpaired Student’s t-test.](/cms/asset/3aa20e9c-389f-4468-92fa-77d722d6f413/koni_a_2363000_f0002_oc.jpg)
Figure 3. Impact of neutrophil depletion on β-Lap therapeutic efficacy. (a–d) Tumor volumes measurement in MC38, TC-1, 4T1, and EO771 tumor models following neutrophil depletion. The depletion of tumor-infiltrating neutrophils was achieved using anti-Ly6G (clone 1A8) antibody, specifically targeting this neutrophil subset. IgG was employed as the anti-Ly6G control in the 4T1 and EO771 tumor models. C57BL/6 WT mice (n = 5/group) were inoculated subcutaneously (s.c.) with 6 x 105 MC38, 1 x 105 TC-1, or 1 x 106 EO771 cells, while BALB/c WT mice (n = 5/group) received subcutaneous inoculations of 6 x 105 4T1 cells. Tumor-bearing mice were subsequently treated with β-Lap (25 mg/kg, i.v., for MC38 and TC-1, or 18 mg/kg, i.t. For 4T1 and EO771 tumors), 20% HPβCD (Vehicle), anti-Ly6G (clone 1A8) antibody (200 µg, i.p.), or a combination of β-Lap+anti-Ly6G antibody every other day for a total of four times once tumor volumes reached ~80 mm3. Tumor volumes were assessed bi-weekly. Tumor volumes of MC38 (A) and TC-1 (b) in C57BL/6 mice. Tumors volumes of 4T1 in BALB/c mice (c) and EO771 in C57BL/6 mice (d). Data are shown as mean ± SD from two independent experiments. *p < 0.05, **p < 0.01 and ***p < 0.001 determined by Student’s 2-tailed t-test.
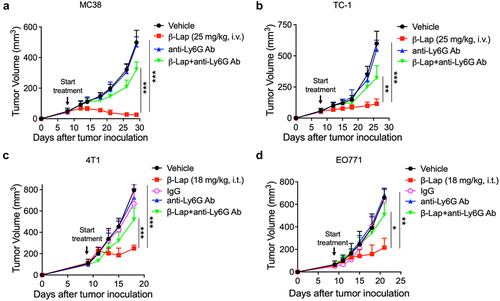
Figure 4. Neutrophils kill murine tumor cells, promote DC recruitment and CD8+ T cell proliferation in the presence of β-Lap-induced antigen medium. (a–d) 4T1 or EO771 cells were exposed to cell culture medium, DMSO, or a lethal dose of β-Lap, for 4 h, followed by washing and medium replacement. After 48 h, the supernatant was collected, yielding cell culture-induced, DMSO-induced, and β-Lap-induced antigen medium, respectively. Bone marrow-derived neutrophils (BMN) were isolated through gradient density centrifugation method. Subsequently, new 4T1 or EO771 cells were co-cultured with the bone marrow-derived neutrophils in the presence or absence of respective antigens for 48 h, after which cell viability was assessed: (a) Representative image of 4T1 cells under microscope. Scale bar = 10 µm (b) Relative survival percentage of 4T1 cells in medium versus in different antigen media as determined by DNA assay. (c) Representative microscope image of EO771 cells. Scale bar = 10 µm (d) Relative survival percentage of EO771 cells determined by DNA assay. (e,f) ROS measurement from BMN culture with DMSO or β-Lap induced antigen medium for 24 h following DCFH-DA staining and flow cytometry acquisition. (g) RT-qPCR analysis for gene related immune cell recruitment (CCL5, CCL4, CCL3, CCL20) and neutrophil activation (INOS and TNF-α). (h) MC38-OVA-bearing mice (n = 3/group) were treated with β-Lap (15 mg/kg, i.t.) for one dose, and 4 days later, CD11b+Gr-1high cells were purified from the tumor drain lymph nodes, and co-cultured with CD8+ T cells isolated from the spleen of OT-1 transgenic mice. The activity of cross-priming of T cells was determined by the level of cell-secreted IFNγ via Cytometric Bead Array (CBA) mouse IFNγ assay. Naïve CD8+ T cells, isolated from the spleen of C57BL/6 OT1 mice and labeled with CFSE, were co-cultured for 3 days with bone marrow-derived neutrophils, which were either exposed to β-Lap-induced antigen medium (prepared by treating MC38-OVA cells with a lethal concentration of β-Lap or control medium: (i) Representative flow cytometry plot of OT1 CD8+ T cell proliferation. (j) Quantification of OT1 CD8+ T proliferation observed in I. Data are presented as mean ± SD from two independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 determined by Student’s 2-tailed t-test.
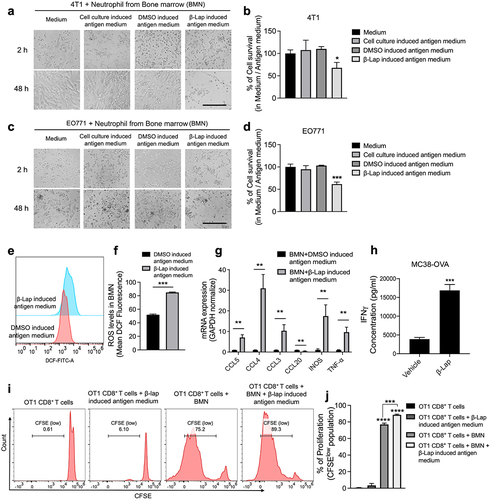
Figure 5. β-Lap treatment induces polarization of tumor associated neutrophils (TANs) towards an anti-tumor (N1) phenotype. 4T1 cells were exposed to DMSO or a lethal dose of β-Lap, for 4 h, followed by washing and medium replacement. After 48 h, the supernatant was collected, yielding β-Lap-induced antigen medium. High density neutrophils (HDN) from bone marrow of BALB/c mice were isolated through a gradient density centrifugation method and then cultured in DMSO induced antigen medium or β-Lap-induced antigen medium for 6 h or 24 h. mRNA expression levels of IFN-β, TGF-β, Fas (CD95) and ICAM1 (CD54) for (a) 6 h and (b) 24 h. (c–f) Flow cytometry was employed to analyze the expressions of cytokines at a time point of 24 h, including IFN-β (c), TGF-β (d), and surface markers CD95 (e) and CD54 (f), which are associated with anti-tumor neutrophils (N1). (g-j) BALB/c WT mice (n = 3/group) were inoculated subcutaneously (s.c.) with 6x105 4T1 cells. Once tumor volumes reached ~80 mm3, tumor-bearing mice were subsequently treated with β-Lap (18 mg/kg, i.t.) or 20% HPβCD (Vehicle) every other day for a total of four treatments. Tumor samples were harvested 24 h after the last administrated dose. The expression of N1 neutrophils-associated markers in TANs was assessed via flow cytometry: (g) Cytokine IFN-β expression, (h) TGF-β expression, (i) Surface markers CD95 expression, and (j) Surface marker CD54 expression. Data are presented as mean ± SD from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 determined by unpaired Student’s t-test.
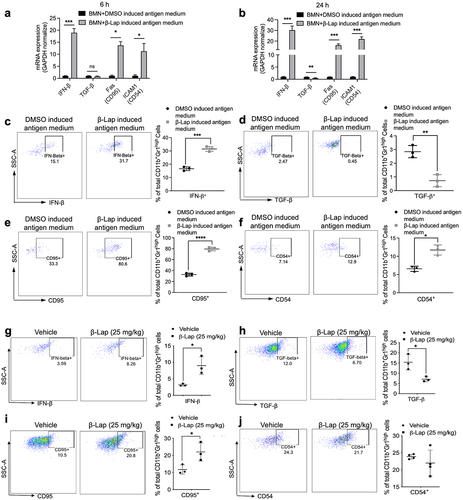
Figure 6. TLR4/MyD88 signaling deficiency or HMGB1 blockade significantly decreases the β-Lap-induced tumor neutrophil infiltration. (a–c) C57BL/6 WT, MyD88−/−, TLR4−/−, or Rag1−/- mice (n = 5/group) were inoculated subcutaneously (s.c.) with 6x105 MC38 cells. Tumor-bearing mice were subsequently treated with β-Lap (25 mg/kg, i.t.) or 20% HPβCD (Vehicle) every other day for a total of four cycles once tumor volumes reached ~80 mm3. Tumor samples were harvested 24 h after the last administrated dose, and tumor-infiltrating neutrophils in the tumor microenvironment were assessed via flow cytometry: (a) Neutrophil populations in MyD88−/− background tumors, (b) Percentage of neutrophils in TLR4−/− mutant tumors, and (c) Percentage of neutrophils in Rag1−/− mutant tumors. (d) Induction of HMGB1 release in the medium after 4 h of β-Lap treatment in MC38 and TC-1 cells was assessed by western blot analysis. (e) MC38 tumor models were established in C57BL/6 WT or TLR4−/− mice, following which mice were treated as described in (a). After the last administered dose, mice were treated with thioglycollate broth for 4 hours, and tumor samples were collected. The migration of peritoneal neutrophils was analyzed in MC38 cell culture medium treated with β-Lap (4 μM) or β-Lap+anti-HMGB1. (f) MC38 tumor models were established as described in (a), and then mice were treated with β-Lap ± anti-HMGB1. Anti-HMGB1 neutralized antibody (200 μg, i.p.) was administrated every 3 days for three times during the treatment. Tumor samples were harvested 24 h after the last administrated dose of β-Lap, and tumor-infiltrating neutrophils in the tumor microenvironment were assessed using flow cytometry. Data are presented as mean ± SD from two independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns: not significant determined by unpaired Student’s t-test.
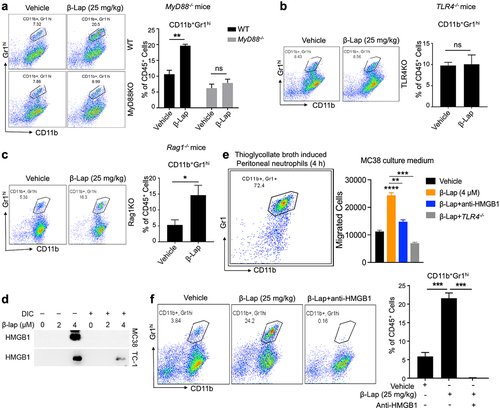
Figure 7. HMGB1 blockade, neutrophils depletion, or TLR4/MyD88 deficiency significantly decreases the antigen-specific T cell response. C57BL/6 WT, TLR4−/−, or MyD88−/− mice (n = 5/group) were transplanted with 6 × 105 MC38 (a, c, e) or 1 × 106 MC38-OVA (b, d, f) cells and treated with β-Lap (25 mg/kg, i.v.) or 20% HPβCD (Vehicle) every other day for three times once tumor volumes reached ~80 mm3. 3 days after the last treatment, lymphocytes from the spleens and tumor-draining lymph nodes (TDLN) were isolated and stimulated in vitro with medium, MC38 cells irradiated with 60 Gy, or stimulated with 2.5 μg/mL of OT1 peptide for 3 days. The production of IFNγ by these stimulated cells was quantified using an ELISPOT assay. Data are presented as mean ± SD from two independent experiments. **p < 0.01, ***p < 0.001 determined by two-way ANOVA.
Supplemental Material
Download MS Word (11.8 MB)Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Data and materials are available on reasonable request.