Figures & data
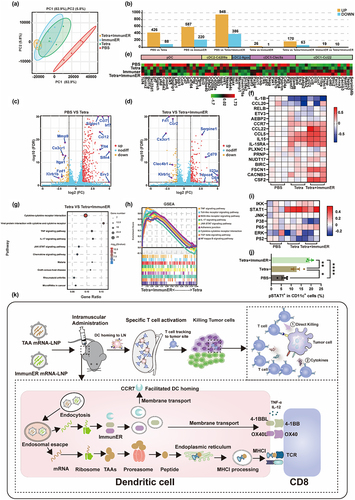
Figure 1. PCa TAA mRNA-LNPs-stimulated immunity suppresses prostate tumor growth in mice.
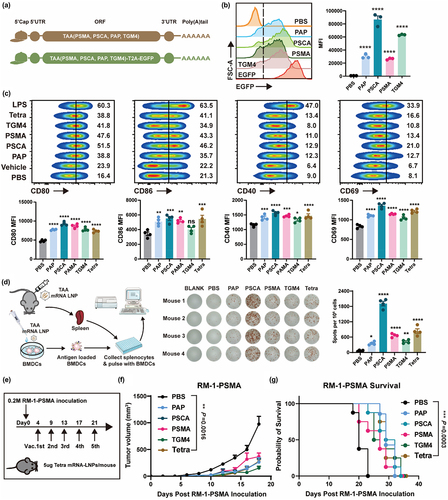
Figure 2. ImmunER enhances the function of dendritic cells and suppresses tumor growth.
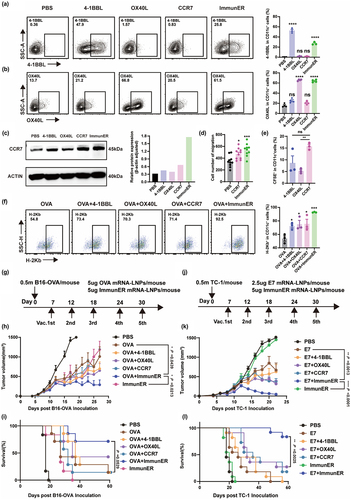
Figure 3. ImmunER enhances the level of immunity in vivo.
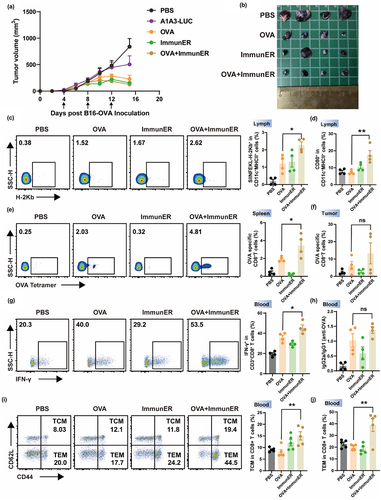
Figure 4. Tetra combined with ImmunER enhances the function of BMDCs in vitro.
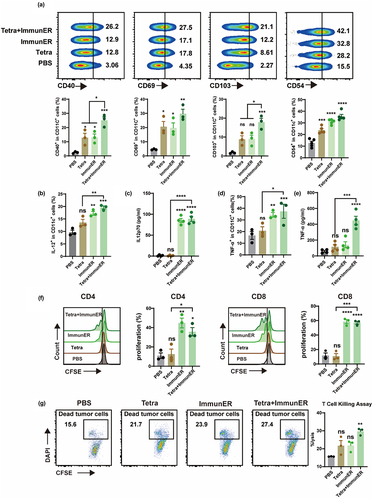
Figure 5. Tetra-ImmunER exhibits therapeutic effects and alters the tumor immune microenvironments in a PCa murine model.
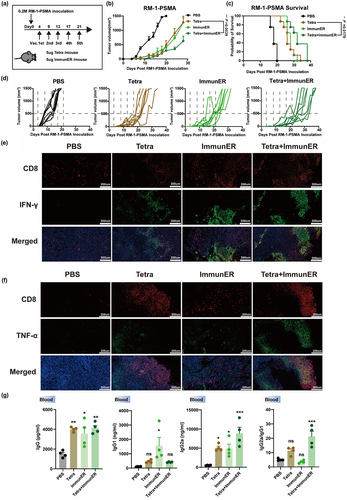
Figure 6. PCa Tetra-ImmunER upregulates cell adaptive immunity.
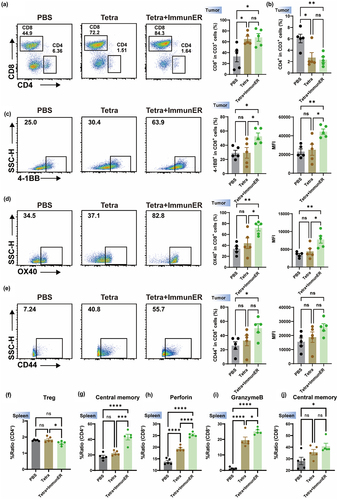
Figure 7. Sequencing of mRNA reveals the upregulation of cDC1-Ccl22 related genes and STAT1.