Figures & data
Figure 1. S100A10 deficiency prevents lung metastasis.
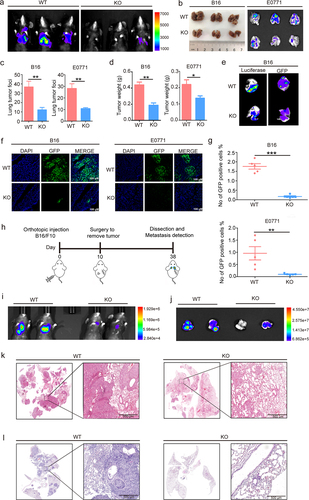
Figure 2. S100A10 deficiency prevents lung pre-metastatic microenvironment formation.
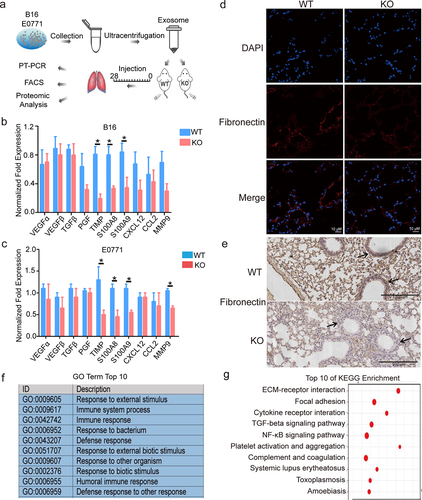
Figure 3. S100A10 deficiency inhibits MDSCs recruitment to the lung pre-metastatic microenvironment.
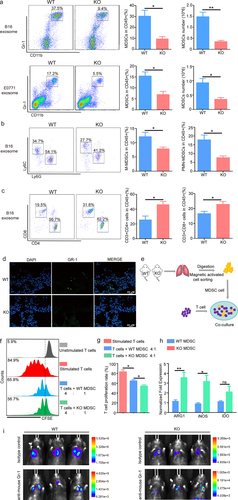
Figure 4. The S100A10 of lung fibroblasts activates CXCL1/CXCL8 expression and increases the migration of myeloid lineage via B16/F10-derived exosome induction.
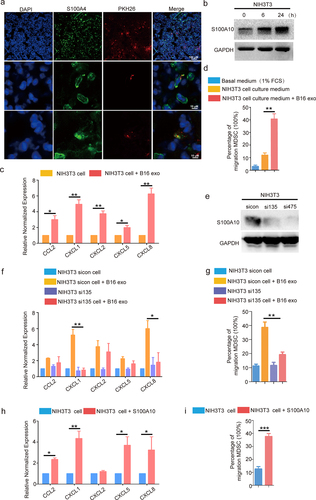
Figure 5. Upregulation of S100A10 expression in lung fibroblasts promotes lung metastasis of tumor in KO mice.
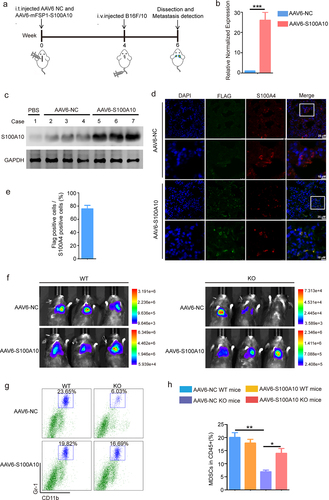
Figure 6. S100A10 inhibitor prevents lung metastasis.
Supplemental Material
Download MS Word (10.2 MB)Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
