Figures & data
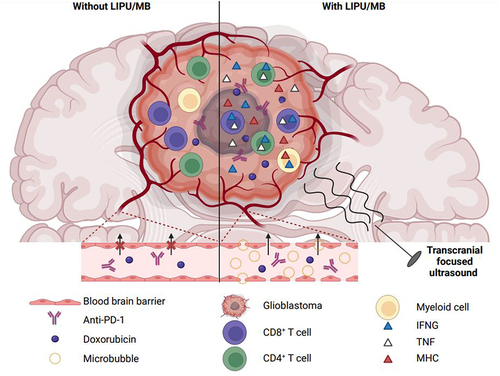
Figure 1. Ultrasounds combined with administration of microbubbles facilitate the delivery of doxorubicin/anti-PD-1 to glioblastoma and improve therapeutic efficacy. LIPU/MB transiently opens the blood-brain barrier, facilitating the access of liposomal doxorubicin and anti-PD-1 to glioblastoma. Locally, concentration of the dual therapeutic agents stimulates IFNG production by cerebral myeloid cells and upregulation of MHC molecules by surrounding cells like malignant cells. This pro-inflammatory environment enhances the recognition of cancer cells by T lymphocytes. These latter show polyfunctionality, secreting both IFNG and TNF, improved antitumor activity, and persist in treated mice surviving the disease. IFNG, interferon-gamma; LIPU, low-intensity pulsed ultrasound; MB, microbubble; MHC, major histocompatibility complex; PD-1, programmed cell death 1; TNF, tumor-necrosis factor-alpha.