Figures & data
Figure 1. Schematic process for preparing the microneedle array reverse mold (MARM) with PDMS using photolithography and reactive ion etching (RIE) methods of the microelectromechanical systems (MEMS) technology.
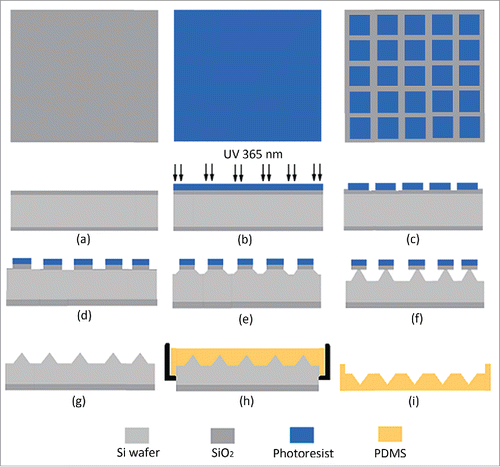
Figure 2. A photo of a microneedle array inverse mold (MAIM) made of PDMS taken in zoom lens with a digital camera (left) and the image of the microholes of a MARM observed under an optical microscope (right). Reprinted with permission from Reference 16.
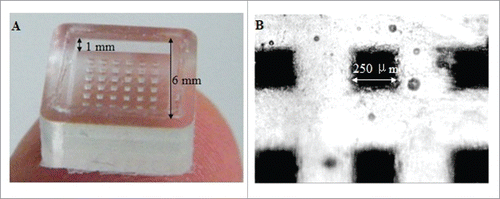
Figure 3. Different type of MAs made of silicon, metal and polymer with microneedles of different shapes. Reprinted with permission from Reference 7.
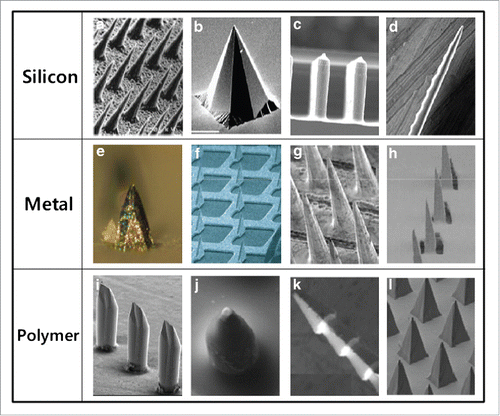
Figure 4. Images of the 3 different microneedle arrays used in this study. A: 300A microneedle array, assembled of 30 G needles. B: 300ED stainless steel microneedle array. C: Dermastamp consisting of 6 microneedles. In figure D, E and F higher magnification images of single microneedles are shown. Reprinted with permission from Reference 32.
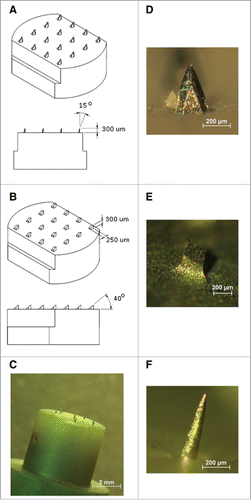
Figure 5. (a) Schematic illustration of (Poly-1/ICMV) multilayers deposited onto PLGA microneedle surfaces (Poly-1 = PBAE). ICMV lipid nanocapsules are prepared with interbilayer covalent cross-links between maleimide head groups (M) of adjacent phospholipid lamellae in the walls of multilamellar vesicles. (Poly-1/ICMV) PEMs were constructed on microneedles after (PS/SPS) base layer deposition. (b) Microneedles transfer (Poly-1/ICMV) coatings into the skin as cutaneous depots at microneedle insertion points. (c) Hydrolytic degradation of Poly-1 leads to PEM disintegration and ICMV release into the surrounding tissue. (d) ICMV delivery to skin-resident APCs provides coincident antigen exposure and immunostimulation, leading to initiation of adaptive immunity. Reprinted with permission from Reference 49.
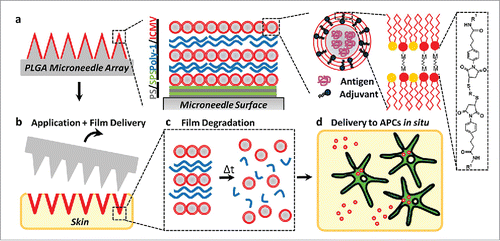
Figure 6. Characteristics of the prepared proMMA and MLLs. (A) Image of the prepared proHMA with 6 × 6 microneedles. (B) An optical microscopy image of a proHMA microneedle, which, upon rehydration, dissolved rapidly with changing its shape within 5 s (C) and almost disappeared in 1 min (D). (E) SEM of the powders of the proHMA microneedles. The within numerous nanospheres were proliposomes of the HBsAg-MLLs. (F) TEM of the HBsAg-MLLs prepared by procedure of emulsion-lyophilization. Reprinted with permission from Reference 16.
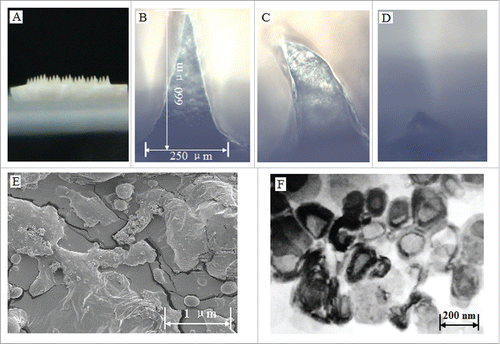
Figure 7. (a) Confocal microscopy images of PLGA-PAA composite microneedles fabricated to encapsulate DiD-loaded PLGA microparticles (MP) (right, scale bar 200 μm). SEM images of (b) resulting microparticle-encapsulating microneedle array (scale bar 200 μm) and (c) high magnification image of the composite needle interior of a fractured microneedle (scale bar 10 μm). Reprinted with permission from Reference 64.
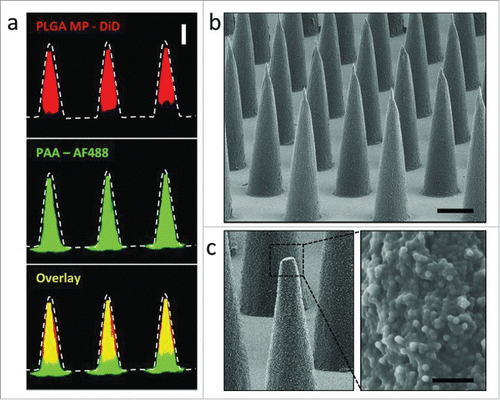
Figure 8. Different VLP platforms developed to produce VLP vaccines with different configurations. Reprinted with permission from Reference 71.
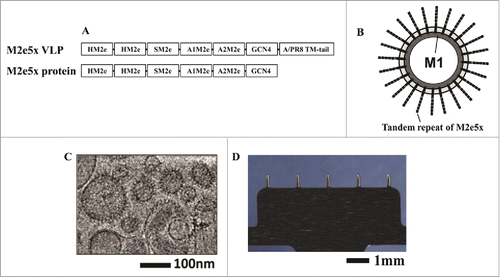
Figure 9. MAs and M2e5x VLPs or M2e5x proteins for vaccination. (A) Structure of M2e5x VLP or M2e5x proteins. HM2e: human M2e, SM2e: swine M2e (2009 pandemic flu), A1M2e: major avian M2e, A2M2e: minor avian M2e. (B) Schematic diagram of influenza M2e5x VLPs containing tandem repeat of heterologous M2e and matrix (M1) proteins. (C) Cryo-TEM (transmission electron microscopy) image of influenza M2e5x VLPs. (D) Microneedle array coated with M2e5x VLPs. Reprinted with permission from Reference 77.