Figures & data
Table 1. Genetically modified proteins in nature and engineered
Figure 1. Molecular model of the alkaline phosphatase CmAP (left) and atomic structure of the wild type CmAP active center (above) and its mutant Tre118Gly (below) in silico generated with program MOE 2012.10.Citation47 The coordinates of the metal ions produced by superposition model CmAP with crystal structure of VAP (PDB: 3E2D). Water is shown as red sphere. Metal ions Zn2+ and Mg2+ were shown as blue and brown spheres, respectively. Hydrogen bonds were shown as discrete lines. The residues Tre118 (above) and Gly118 (below) were shown as full and empty cursor, respectively.
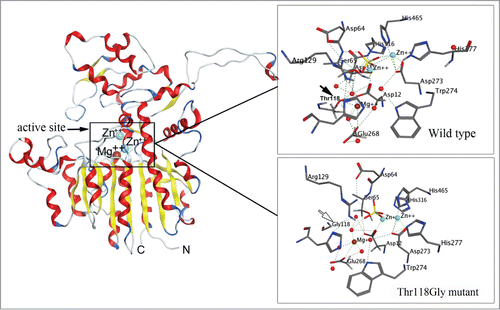
Figure 2. 2D-diagram of the model oligosaccharide binding with the single mutant A137N of the Far Eastern holothurian mannan-binding lectin MBL-AJ and its contacts with the oligosaccharide (left). 2D-diagram of the model oligosaccharide binding with the MBL-AJ double mutant F159K and its contacts with the oligosaccharide (right). The residue substitution positions 137 and 159 were indicated by double circles.
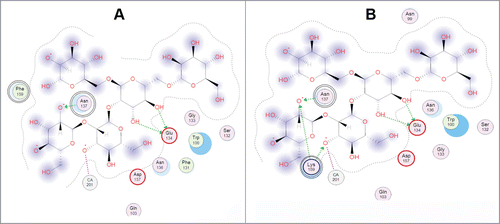
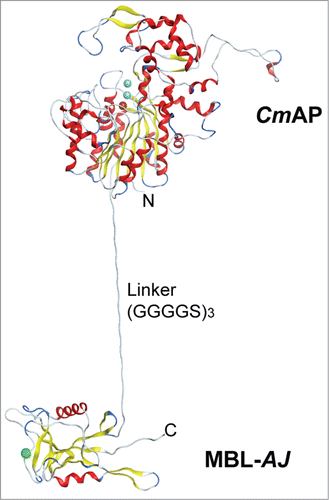
Figure 3. The structural model of the Far Eastern holothurian mannan-binding hybrid lectin MBL-AJ monomer with the alkaline phosphatase CmAP (CmAP/MBL-AJ). C-terminal end of the CmAP subunit is linked via flexible linker (G4S)3 with N-terminal end of the lectin MBL-AJ subunit that allow to function domains independently from each other.Citation44 Three spheres in CmAP module indicate 2 ions Zn2+ and one ion Mg2+; one sphere in MBL-AJ indicates ion Ca2+. α-Helixes and β-strands are shown as ribbons. Unordered structures are shown as threads.