Figures & data
Figure 1. SDS-PAGE analysis of recombinant wild-type UGT88A1 and mutant V18R preparations. Lane 1: crude extract obtained from E. coli BL21(DE3) without plasmid; Lane 2: standard molecular marker; Lane 3: crude extract obtained from the recombinant strain E. coli BL21(88A1) with induction; Lane 4: purified wild-type UGT88A1; Lane 5: crude extract obtained from the recombinant strain E. coli BL21(V18R) with induction; Lane 6: purified mutant V18R.
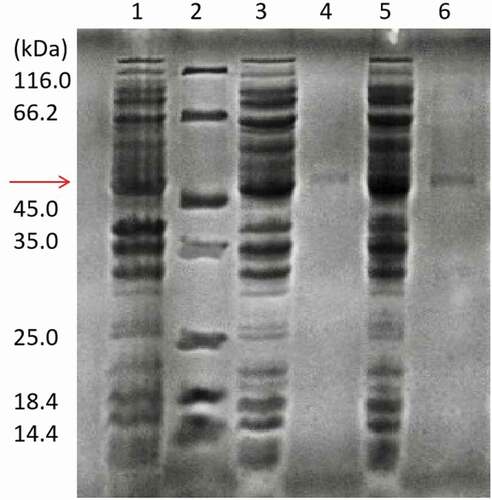
Figure 2. pH effect on the enzyme activity (a), and the enzyme stability for producing quercetin-3-O-glucoside (b) and quercetin-4′-O-glucoside (c). Que-3, Quercetin-3-O-glucoside; Que-4′, quercetin-4′-O-glucoside.
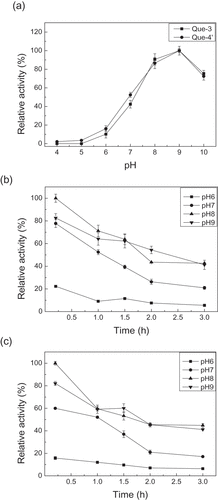
Figure 3. Effect of temperature on the enzyme activity (a), and the enzyme stability for producing quercetin-3-O-glucoside (b) and quercetin-4′-O-glucoside (c). Que-3, Quercetin-3-O-glucoside; Que-4′, quercetin-4′-O-glucoside.
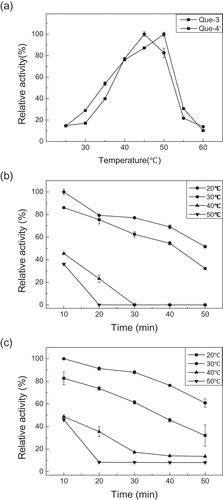
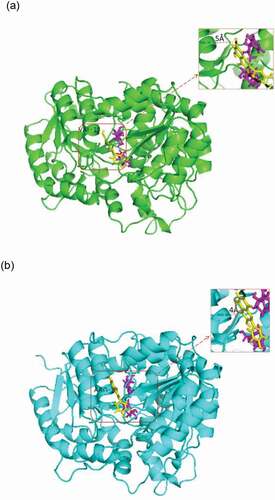
Figure 4. Protein sequence and structure analysis of UGT88A1. (a) Part of the multiple alignment of nine UGTs by Clustal O. ‘*’, ‘:’, and ‘.’, respectively, indicate the positions that have a single, fully conserved residue, and the residue with the ‘strong’ or ‘weaker’ groups fully conserved. (b) Stereo diagram of Val-18 and the atoms around (His-16 and quercetin). Quercetin is shown as a stick model.
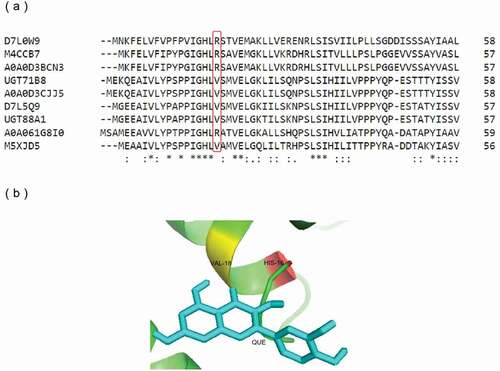
Table 1. Specific enzyme activities and kinetic parameters of wild-type UGT88A1 and mutant V18R for producing quercetin-4′-O-glucoside.