Figures & data
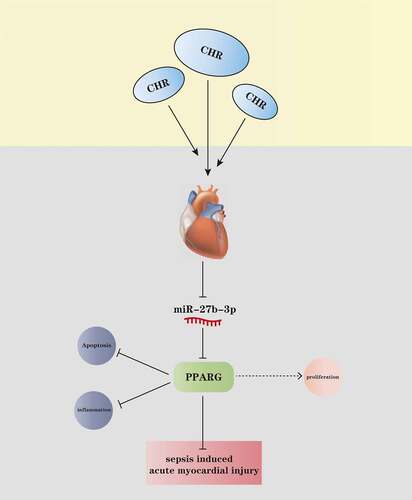
Table 1. The sequence of each molecular primer
Figure 1. CHR defended cardiomyocytes from LPS damage Different concentrations of CHR (1, 5, 25, 50, 100 μ M) was dealt with H9c2 cells. A: cell viability was detected by MTT. H9C2 cells were treated with LPS (10–25 μg/ml) for 24 h to induce an in-vitro model of S-AMI. B: MTT examined cell viability; C: Edu assay detected cell viability. CHR was utilized to treat H9C2 cells at different concentrations (20 μM, 40 μM, 80 μM). D: Flow cytometry monitored cell apoptosis; E-H: ELISA measured pro-inflammatory factors’ levels in H9C2 cells; I: Western blot was taken to analyze the profiles of pro-inflammatory proteins NF-κB-p65, MAPK-p38, JNK1/2 in H9C2 cells. NS P > 0.05,**P < 0.01, ***P < 0.001 (vs. the control group); #P < 0.05, ##P < 0.01, ###P < 0.001 (vs. the LPS group). N = 3.
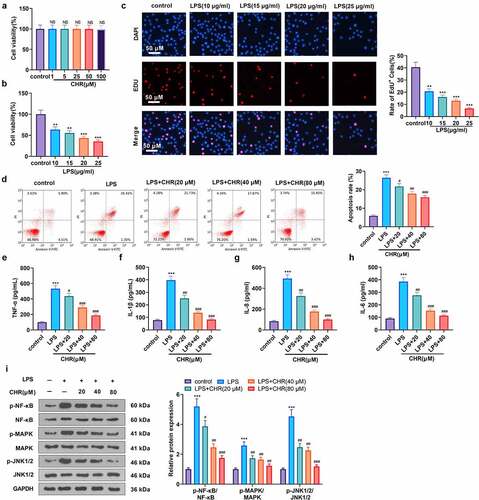
Figure 2. CHR attenuated myocardial damage in sepsis rats A CLP surgery was performed on rats for constructing a rat sepsis model. CHR was administered at different doses (25, 50, 100 mg/kg). A: ELISA was used for evaluating serum myocardial injury markers (CK-MB, cTnI, BNP). B-F: cardiac output (CO), ejection fraction (EF), shortening fraction (FS), left ventricular end diastolic diameter (LVEDD) and left ventricular end-systolic diameter (LVESD) were measured by echocardiography 24, 48 and 72 hours after CLP. G: HE staining was preforming the pathological changes of myocardial tissues. H: TUNEL assay was used for evaluating cardiomyocyte apoptosis. J: MPO-staining was used for detecting neutrophil infiltration using immunohistochemistry, and the MPO activity in the heart was detected. ***P < 0.001 (vs. Sham group); NS P > 0.05, #P < 0.05, ##P < 0.01, ###P < 0.001 (vs. CLP group). N = 5.
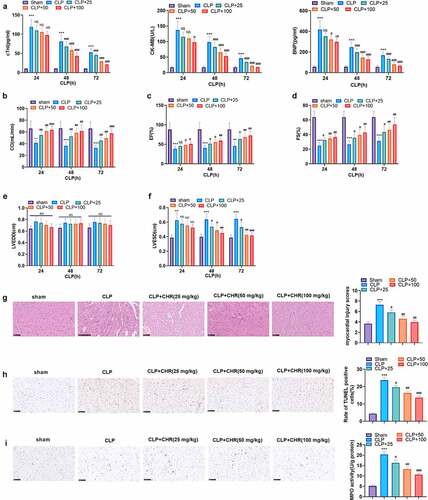
Figure 3. CHR suppressed miR-27b-3p and enhanced PPARG H9c2 cells were treated with LPS (10 μg/ml) for 24 hours to construct the cell septic injury model. A-B: qRT-PCR was used to detect the expression of miR-27b-3p and PPARG in H9c2 cells 24, 48, 72 and 96 hours after LPS treatment. Different concentrations of Chr (20, 40, 80 μ M) were added into the H9c2 cells. C-D. The expression of miR-27b-3p and PPARG was detected by qRT-PCR. E: The expression of PPARG was detected by cellular immunofluorescence. Scale bar = 50 μm. F: The expressions of c-Myc and PPARG were detected by western blot. ** P < 0.01, ***P < 0.001(vs. control group); # P < 0.05, ##P < 0.01, ###P < 0.001 (vs. LPS group). N = 3.
Figure 4. miR-27b-3p targeted PPARG A: The Starbase database (http://starbase.sysu.edu.cn/) was introduced for predicting the base binding correlation between miR-27b-3p and PPARG; B: H9C2 cells were transfected along with the luciferase reporter vectors (PPARG-WT and PPARG-MUT) with miR-NC or miR-27b-3p mimics. Dual-luciferase activity assay evaluated the luciferase activity of H9C2 cells to confirm the correlation between miR-27b-3p and PPARG. C: The expression of PPARG was detected by cellular immunofluorescence. Scale bar = 50 μm. D: Western blot was conducted to evaluate the protein profile of PPARG in H9C2 cells transfected with miR-NC or miR-27b-3p mimics. NS P > 0.05, ***P < 0.001 (vs. the miR-NC group). N = 3.
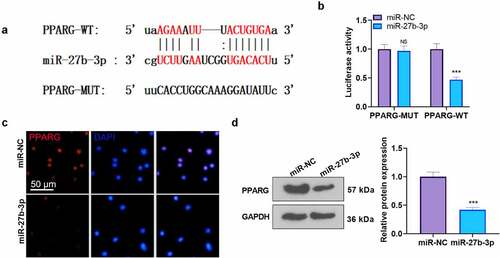
Figure 5. CHR protected cardiomyocytes against LPS-mediated injury by modulating the miR-27b-3p/PPARG axis After the construction of the H9C2 cell injury model with the use of LPS (10 μg/ml), CHR (20 μM) and RGZ (100 nM) were adopted to treat the cells. A. H9C2 cells were transfected along with miR-27b-3p mimics or miR-NC, and miR-27b-3p’s level was gauged via RT-PCR. CHR was administered after miR-27b-3p mimics transfection in H9C2 cells. B: Western blot measured PPARG; C: MTT checked cell viability. D: EdU assay detected cell proliferation. E-F: Flow cytometry checked cell apoptosis, respectively; G-J: ELISA was applied to confirm the profiles of pro-inflammatory factors; K: Western blot verified the levels of pro-inflammatory proteins NF-κB MAPK, and JNK1/2 in H9C2 cells. ***P < 0.001 (vs. the miR-NC group); ***P < 0.001 (vs. the Vector group); **P < 0.01, ***P < 0.001 (vs. the NC group); #P < 0.05, ##P < 0.01 (vs. the LPS group); &&P < 0.01, &&&P < 0.001 (vs. the LPS+miR-27b-3p group). N = 3.
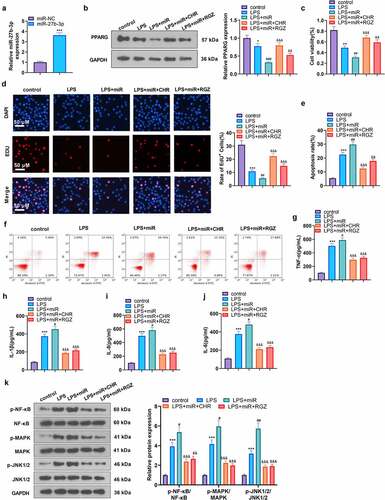
Figure 6. CHR lessened inflammatory damage in sepsis rats Cecal ligation and puncture (CLP) was operated for conducting a sepsis rat model, and the rats were subcutaneously injected with CHR (25, 50, 100 mg/kg). Seventy-two hours later, the rats were sacrificed, and their hearts were collected for histopathological examination. A-D: ELISA was implemented to analyze the levels of pro-inflammatory factors (IL-8, TNF-α, IL-6, IL-1β) in the rats; E: Western blot examined the levels of pro-inflammatory proteins NF-κB, MAPK, and JNK1/2. ***P < 0.001 (vs. the Sham group); #P < 0.05, ##P < 0.01, ###P < 0.001 (vs. the CLP group). N = 5.
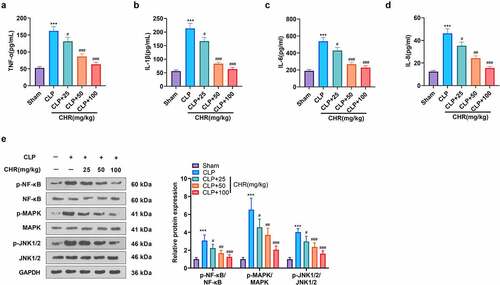
Figure 7. CHR attenuated miR-27b-3p and boosted PPARG expression in the sepsis rat heart Cecal ligation and puncture (CLP) was performed to engineer a sepsis rat model, and the rats were subcutaneously injected with CHR (25, 50, 100 mg/kg). Seventy-two hours later, the rats were sacrificed, and their hearts were harvested for further examination. A. RT-PCR tested miR-27b-3p expression in the heart. B. Western blot examined c-Myc and PPARG in the heart. ***P < 0.001 (vs. the Sham group); #P < 0.05, ##P < 0.01, ###P < 0.001 (vs. the CLP group). N = 5.
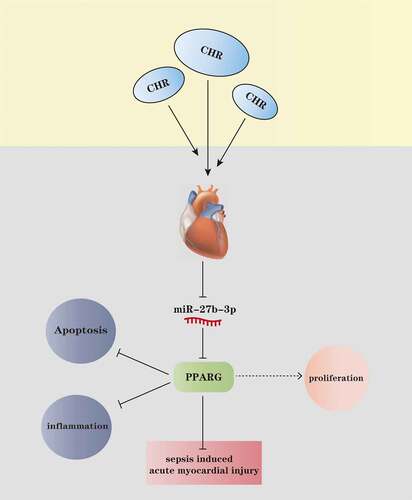
Figure 8. The mechanism diagram of CHR CHR treatment elevated the expression level of PPARG by repressing miR-27b-3p in the myocardium, thereby lessening myocardial cell apoptosis and inflammatory factor release, enhancing cell viability, and ultimately impeding acute myocardial injury elicited by sepsis.
Data availability statement
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
