Figures & data
Figure 1. LGG elevated IL-22 expression and affected alcohol-induced liver functions. (a) The mRNA expression of IL-22 of liver tissues was evaluated by qRT-PCR in control, model, model+LGG, and LGG groups. The 8- to 10-week-old C57BL/6 J mice were used to construct chronic plus binge alcoholic liver disease model. For LGG treatment, mice received live LGG by gavage at a dose of 109 colony-forming units every day for 15 days. The control mice were fed with the same volume of physiological saline. (b) The level of IL-22 in serum was detected by ELISA. (c-e) Levels of ALT (c), AST (d), and TG (e) in serum of mice with different treatments. **p < 0.01, ***p < 0.001 vs. Control; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001 vs. model; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. model+LGG. LGG, Lactobacillus rhamnosus GG; IL-22, interleukin 22; qRT-PCR, quantitative real-time polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay; ALT, alanine aminotransferase; TG, triglyceride; AST, aspartate aminotransferase.
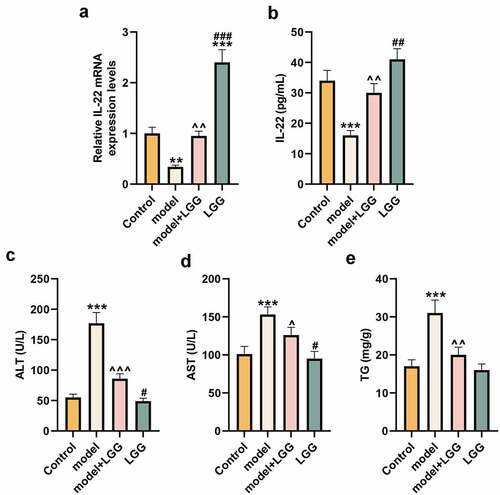
Figure 2. LGG alleviated injury of liver and intestinal barrier caused by alcohol. (a) Representative HE staining of liver tissues in different treatments, alcohol treatment resulted in liver injury, inflammatory infiltration, steatosis and lipid droplets elevated, while LGG could reduced this liver injury. Magnification, ×400; Scale bar, 100 μm. (b) Representative HE staining of colon tissues in different treatments. Magnification, ×40; Scale bar, 100 μm. (c-d) Protein levels of ZO-1 and Claudin-1 in colon tissues were determined by western blot analysis. (c) Representative protein bands. (d) Quantification of proteins. The 8- to 10-week-old C57BL/6 J mice were used to construct a chronic plus binge alcoholic liver disease model. For LGG treatment, mice received live LGG by gavage at a dose of 109 colony-forming units every day for 15 days. The control mice were fed with the same volume of physiological saline. ***p < 0.01 vs. Control; ^p < 0.05, ^^p < 0.01 vs. model; ##p < 0.01 vs. model+LGG. LGG, Lactobacillus rhamnosus GG; HE, hematoxylin eosin; ZO-1, tight junction protein 1.
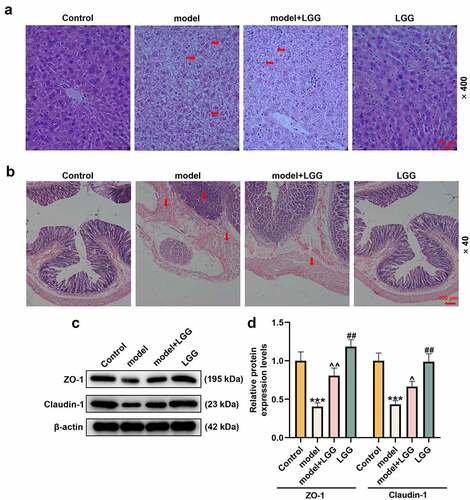
Figure 3. LGG affected alcohol-induced liver damage by regulating IL-22 expression. (a) The mRNA expression of IL-22 in liver tissues was detected by qRT-PCR after downregulation of IL-22. (b-d) Levels of ALT (b), AST (c), and TG (d) in serum of mice with different treatments. The 8- to 10-week-old C57BL/6 J mice were used to construct a chronic plus binge alcoholic liver disease model. For LGG treatment, mice received live LGG by gavage at a dose of 109 colony-forming units every day for 15 days. One dose of Ad-shRNA-NC (shNC, 1 × 109 PFU/mouse, i.v.) and Ad-shRNA-IL-22 (1 × 109 PFU/mouse, i.v.) was given on the day prior to ethanol feeding. *p < 0.05, **p < 0.01, ***p < 0.001 vs. model+LGG+shNC; &p < 0.05, &&p < 0.01, &&&p < 0.001 vs. shNC. LGG, Lactobacillus rhamnosus GG; IL-22, interleukin 22; qRT‐PCR, quantitative real-time polymerase chain reaction; ALT, alanine aminotransferase; TG, triglyceride; AST, aspartate aminotransferase; NC, negative control, Ad, adenoviral vector.
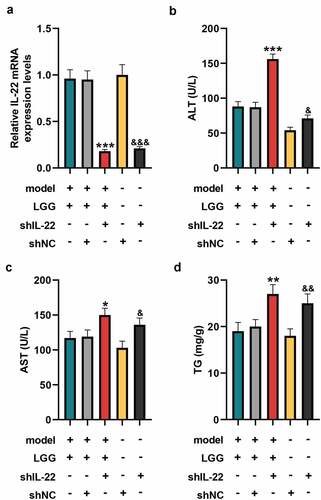
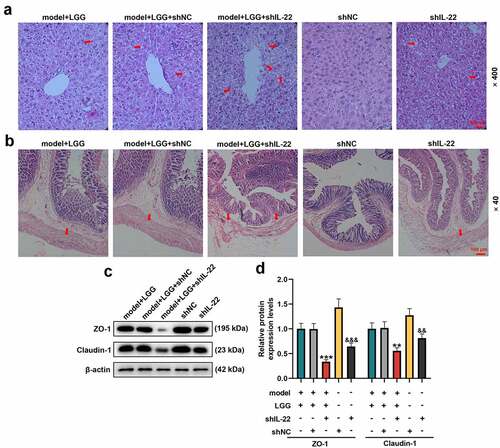
Figure 4. LGG alleviated alcohol-induced injury of liver and intestinal barrier through regulation of IL-22 expression. (a) Representative HE staining of liver tissues in different treatments. Magnification, ×400; Scale bar, 100 μm. (b) Representative HE staining of colon tissues in different treatments. Magnification, ×40; Scale bar, 100 μm. (c-d) Protein levels of ZO-1 and Claudin-1 in colon tissues were determined by western blot analysis. (c) Representative protein bands. (d) Quantification of proteins. The 8- to 10-week-old C57BL/6 J mice were used to construct a chronic plus binge alcoholic liver disease model. For LGG treatment, mice received live LGG by gavage at a dose of 109 colony-forming units every day for 15 days. One dose of Ad-shRNA-NC (shNC, 1 × 109 PFU/mouse, i.v.) and Ad-shRNA-IL-22 (1 × 109 PFU/mouse, i.v.) was given on the day prior to ethanol feeding. **p < 0.01, ***p < 0.001 vs. model+LGG+shNC; &&p < 0.01, &&&p < 0.001 vs. shNC. LGG, Lactobacillus rhamnosus GG; HE, hematoxylin eosin; ZO-1, tight junction protein 1.
Data availability statement
The analyzed data sets generated during the study are available from the corresponding authors on reasonable request.
