Figures & data
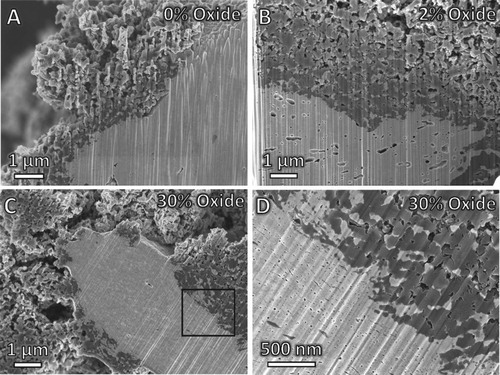
Figure 1. (A) Carbon deposition quantities for each sample after 1 hr under 4:1 C2H4:H2 at listed temperatures, and (B) increase in porosity after 1 hr under 5% H2 (bal. Ar) at listed temperatures.
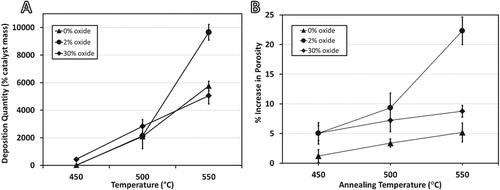
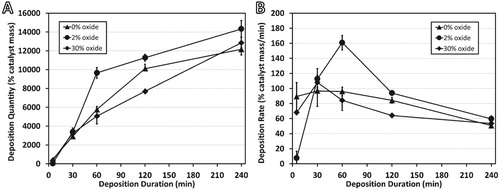
Figure 2 (A) Total carbon deposition and (B) carbon deposition rates measured for samples at reaction times shown. All reactions performed at 550°C under 4:1 C2H4:H2.