Figures & data
Figure 1 Change in ALSFRS-R total score for subgroups of participants according to pre-study rate of progression in FORTITUDE-ALS. Analysis includes all participants with an ALSFRS-R total score measured at baseline and at least one timepoint after baseline. ALSFRS-R: Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised; FPT: fastest-progressing tertile; LS: least squares; IPT: intermediate-progressing tertile; SPT: slowest-progressing tertile. Reprinted by permission of the publisher (Taylor & Francis. Ltd, http://www.tandfonline.com) from Shefner et al. [Citation9]
![Figure 1 Change in ALSFRS-R total score for subgroups of participants according to pre-study rate of progression in FORTITUDE-ALS. Analysis includes all participants with an ALSFRS-R total score measured at baseline and at least one timepoint after baseline. ALSFRS-R: Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised; FPT: fastest-progressing tertile; LS: least squares; IPT: intermediate-progressing tertile; SPT: slowest-progressing tertile. Reprinted by permission of the publisher (Taylor & Francis. Ltd, http://www.tandfonline.com) from Shefner et al. [Citation9]](/cms/asset/39f3d07f-4c95-44f8-a069-5fb98382e524/iafd_a_2216223_f0001_c.jpg)
Table 1 Change in ALSFRS-R total score in FORTITUDE-ALS, showing (A) the overall study population and (B) the subgroup with symptom onset ≤24 months and baseline ALSFRS-R total score ≤44.
Figure 2 COURAGE-ALS study design schematic. Participants completing week 48 may enter a planned open-label extension instead of the 4-week follow-up. ALSAQ-40: Amyotrophic Lateral Sclerosis Assessment Questionnaire; ALSFRS-R: Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised; EQ-5D-5L: EuroQol-5-dimension-5-level; EQ-VAS: EuroQol Visual Analogue Scale; FVC: forced vital capacity
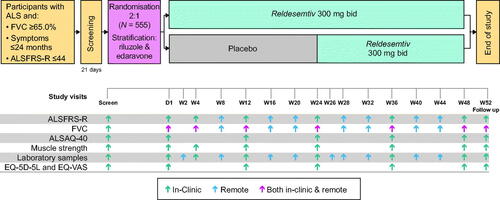
Table 2 COURAGE-ALS key inclusion criteria.
Table 3 COURAGE-ALS key exclusion criteria.
Table 4 Dates for milestones of DME use in COURAGE-ALS.
Table 5 COURAGE-ALS study endpoints.
Data availability statement
Data reported herein are part of a sponsor-led clinical development program that is ongoing, and thus study protocols and complete datasets for the trial will not be made available with this report.
