Figures & data
Table 1. Spindle position and cellular fates in different mammalian epithelial tissues
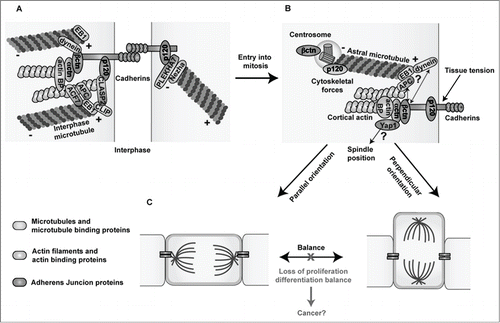
Figure 1. Links between Adherens Junctions, centrosomes and the cytoskeleton during interphase and cell division. (A) In interphase, classical cadherins mediate homophilic cell-cell adhesion through their extracellular domains. The cadherin cytoplasmic domain binds to β- and p120-catenin, while α-catenin binds to the complex via β-catenin. Cadherin-catenin complexes are linked to the actin and microtubule networks via actin- and microtubule- binding proteins. (B) During cell division, centrosomes duplicate and one of the centrosomes migrates to the other pole of the dividing cell forming the mitotic spindle (not shown), which is anchored to the cell cortex through the astral microtubules. Both β- and p120-catenin are found in centrosomes and at the cell cortex potentially promoting the proper positioning of centrosomes and mitotic spindles by anchoring astral microtubules to the cell cortex through their interaction with microtubule binding proteins. Moreover, through their mechanosensing functions, cadherin-catenin complexes may transmit the forces exerted on astral microtubules pulling the spindles toward their final position, leading to either symmetric or asymmetric divisions through their connections with the actin cytoskeleton and/or the Hippo effector Yap1. (C) A proper balance between symmetric and asymmetric cell divisions needs to be maintained in order to preserve tissue homeostasis. Loss of the expression or functional activity of cadherin-catenin proteins may perturb centrosome organization and the oriented positioning of mitotic spindles rendering cells susceptible to transformation. Microtubules and microtubule binding proteins are shown in blue; actin filaments and actin binding proteins are shown in green, and Adherens Junction proteins are highlighted in orange. Actin BP: actin binding proteins.