Figures & data
Figure 1. Model of HIV penetration into mucosal epithelium through disrupted junctions. (A) Multistratified, nonkeratinized oral squamous mucosal epithelia have well-developed tight and adherens junctions within the strata granulosum, spinosum and parabasal layers. The intact tight junctions seal intercellular spaces, preventing paracellular penetration by HIV. (B) Nonkeratinized ectocervical and vaginal squamous mucosal epithelia also have multilayer organization, but their uppermost few layers lack the tight and adherens junctions, allowing paracellular penetration by HIV into the lower layers. These virions are more likely to reach interepithelial HIV-susceptible CD4+ T lymphocytes, LC/DC and macrophages and initiate systemic HIV infection. (C) Fetal and infant oral mucosal epithelia have paucistratified (2 to 5 layers) epithelia with tight and adherens junctions, which may play a role in preventing HIV mother-to-child transmission. (D) Monostratified columnar epithelia of rectal/intestinal and endocervical mucosa have tight and adherens junctions, which prevent paracellular penetration by HIV. (A–D) HIV transmission via stratified and nonstratified mucosal epithelia with intact tight junctions may occur by viral transcytosis; however, the efficiency of transcytosis is < 0.01% of the initial inoculum. Interaction of HIV with the surface of multistratified, paucistratified and monostratified mucosal epithelia may induce the disruption of epithelial tight junctions, promoting paracellular penetration by HIV and increasing its infectivity in CD4+ lymphocytes, LC/DC and monocyte/macrophages. (C and D) HIV systemic infection could also be initiated by the capture of virions across paucistratified fetal/infant oral and monostratified intestinal mucosa by submucosal LC/DC. GR, granulosum; SP, spinosum; BL, basal; BM, basement membrane.
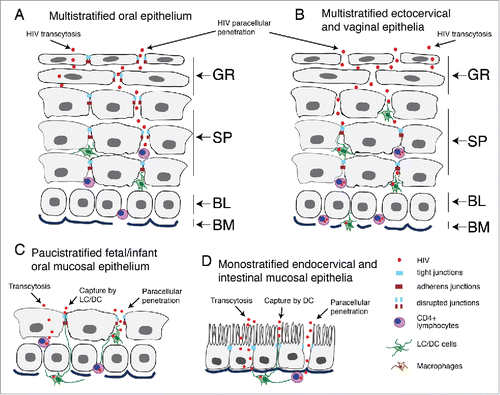
Figure 2. Model of HIV-associated disruption of tight junctions in initial HIV entry and systemic HIV/AIDS disease. (Upper panel) Tight junctions are formed between epithelial cells of oral, genital and intestinal mucosa by lateral interaction of integral membrane proteins occludin and claudins, which are associated with the cytoplasmic proteins ZO-1, ZO-2, and ZO-3. These proteins themselves link occludin and claudins to the actin cytoskeleton. Junctional adhesion molecule 1 (JAM-1) is also localized at the tight junctions of mucosal epithelia. (Lower left panel) HIV-associated disruption of mucosal epithelium may occur upon initial infection of HIV and during systemic HIV/AIDS disease. Initial HIV gp120 interaction with mucosal epithelia is facilitated by binding of viral gp120 to one or more HIV coreceptors (GalCer, CCR5 and/or CXCR4), as well as to HSPG, TLR2 and TLR4. Such interaction activates MAPK, PI3K and/or TLR signaling, leading to upregulation of proinflammatory cytokines, including TNF-α, which induce the activation of MLCK and MLC phosphorylation and actin cytoskeletal contraction. These events induce mislocalization of ZO-1 from junctional areas and internalization of occludin and claudins 1, 3 and 4 from assembled junctions. An increase in the expression of proinflammatory cytokines may activate the apoptotic pathway in epithelial cells, leading to MMP- and/or caspase-mediated degradation of junctional proteins. Disruption of epithelial tight junctions facilitates opening of the paracellular space between epithelial cells and paracellular penetration by HIV. During HIV/AIDS disease, HIV-infected subepithelial CD4+ lymphocytes, LC/DC and macrophages migrate into the mucosal epithelium and release virions and envelope protein gp120, which disrupt epithelial junctions. In addition, HIV-infected cells secrete the viral transactivator protein tat, which also contributes to the disruption of tight junctions. (Lower right panel) HIV tat binds to integrins and induces MAPK activation, which downregulates expression of tight junction proteins ZO-1, occludin, and claudins 1, 3 and 4. The lack of expression of tight junction proteins leads to a lack or incomplete formation of tight junctions.
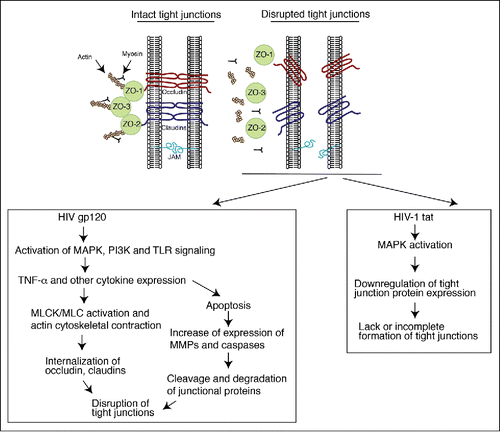
Figure 3. Disruption of tight junctions of HIV-infected oral mucosal epithelia is associated with infiltration of activated lymphocytes, macrophages and natural killer cells expressing proinflammatory cytokines TNF-α and IFN-γ. (A) Buccal biopsy samples from HIV-infected and HIV-uninfected individuals were immunostained for occludin (red). EP, epithelium. (B) Buccal tissues from HIV-infected and HIV-uninfected individuals were coimmunostained for TNF-α or IFN-γ (green) and the markers for T lymphocytes (CD3) and natural killer cells (CD56) (red), respectively. EP, epithelium; LP, lamina propria. (A and B) Cell nuclei were counterstained with TO-PRO-3 iodide (blue). Cells were analyzed using a krypton-argon laser coupled with a Bio-Rad MRC2400 confocal head. The data were analyzed using Laser Sharp software. In panel B, yellow indicates colocalization of TNF-α or IFN-γ immune cell markers. (C) For evaluation of tight junction integrity of oral mucosal epithelium, buccal tissue sections from HIV-infected and HIV-uninfected individuals were immunostained for occludin, and epithelial cells in ring-shaped patterns were counted. For quantitative evaluation of TNF-α-expressing cells, CD3-, CD68-, and CD1a-positive cells were costained for TNF-α. In parallel experiments, CD3- and CD56-positive cells were costained for IFN-γ. Total numbers of TNF-α- and IFN-γ-expressing cells were counted in the mucosal epithelium. Cells were counted in a minimum of 10 separate, randomly chosen fields, and average numbers were presented in mm2. The total number of intraepithelial immune cells expressing TNF-α or IFN-γ was compared with the percentage of epithelial cells expressing a normal pattern of tight junction protein occludin. Statistical analysis was performed using Spearman rank correlation test. Collection of all biopsy tissue samples was approved by the Committee on Human Research of the University of California, San Francisco (IRB approval # H8597-30664-03).
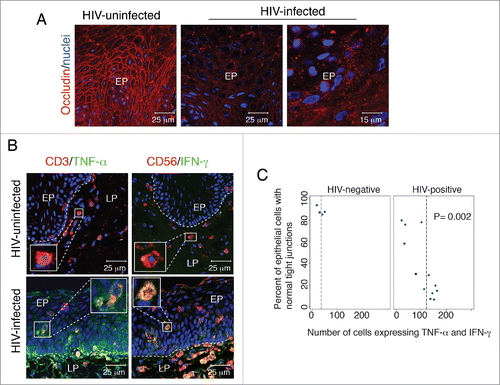
Figure 4. Proinflammatory cytokines TNF-α and IFN-γ disrupt tight junctions of oral epithelial cells. (A) Polarized oral epithelial cells were treated with recombinant TNF-α and IFN-γ at 10 ng/ml each, independently and in combination, for 48 h. Cells were immunostained for the tight junction protein occludin (green). Nuclei are stained in blue. Cells were analyzed by confocal microscopy. (B) (Upper panel) Transepithelial resistance was measured with an epithelial Millicell-ERS voltohmmeter. (Lower panel) Paracellular permeability was evaluated by adding horseradish peroxidase-conjugated goat anti-donkey IgG to the upper compartments of Transwell inserts and, after 1 h, photometrically assaying horseradish peroxidase in the medium from the lower compartment using o-phenylenediamine dihydrochloride as the substrate. The values are expressed as optical density (OD, 450 nm). Detection of IgG in the lower chamber indicated leakage of IgG from the upper chamber via disrupted tight junctions. Error bars show the mean ± SE taken from a total of 3 samples.
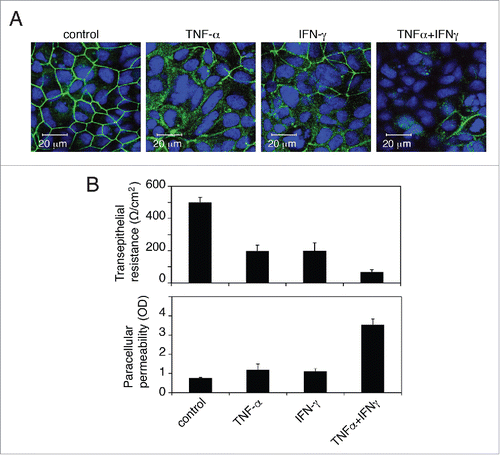
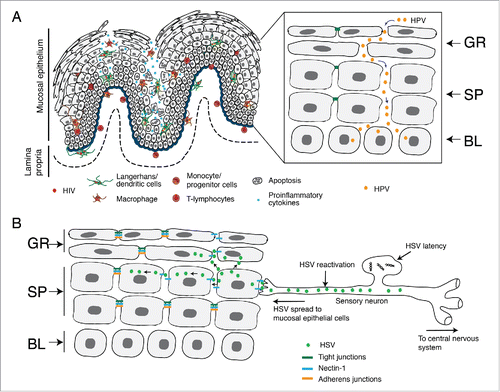
Figure 5. HIV/AIDS-associated disruption of mucosal epithelium facilitates HPV and HSV infection. (A) In HIV-positive individuals, HIV-infected CD4+ T lymphocytes, LC/DC and macrophages infiltrate the mucosal epithelium, releasing virions and viral proteins gp120 and tat. Interaction of virions and viral proteins with one or more HIV coreceptors (CCR5, CXCR4, GalCer, HSPG), TLR-2/4, integrins and/or mannose receptor of epithelial cells induces the activation of MAPK, PI3K, TLR and NF-κB signalings, which leads to the expression of proinflammatory cytokines (TNF-α, IFN-γ, IL-1, IL-1β, IL-2, IL-6, IL-8, and IL-13 ), MMPs (MMP-2 and -9) and caspase (caspase-3 and -6). This mediates the disruption of epithelial tight and adherens junctions by downregulating their expression, aberrant internalization and/or degradation. HIV-associated disruption of tight junctions of oral and genital epithelium facilitates paracellular penetration by HPV toward basal epithelial cells, where HPV initiates lytic infection and the neoplastic process. (B) HIV-associated immune dysfunction leads to the reactivation of HSV-1 from sensory neurons. Reactivated virus infects epithelial cells through the interaction of viral envelope glycoprotein D with its receptor nectin-1. Nectin-1 is sequestered within the intact adherens junctions region of lateral membranes of epithelial cells. HIV-induced disruption of adherens junctions liberates nectin-1 from its sequestered areas, binding HSV glycoprotein D and thereby promoting HSV infection and cell-to-cell spread from neurons to epithelial cells and within the oral epithelium. This leads to the rapid progression of HSV-mediated mucosal lesions and ulcers. GR, granulosum; SP, spinosum; BL, basal.