Figures & data
Figure 1. Changes in cell morphology and actin organization during epithelial scattering. Cells, with plasma membrane in gray, points of adhesion-actin connection as black spots, and actin cables as black lines, undergo a cell spreading phase that is accompanied by rearrangement of the actin cytoskeleton from a circumferential, highly bundled actin organization into transcellular networks. Spread colonies of cells then undergo subsequent actomyosin-based contractility that ruptures cell-cell adhesions and allows detachment of individual cells.
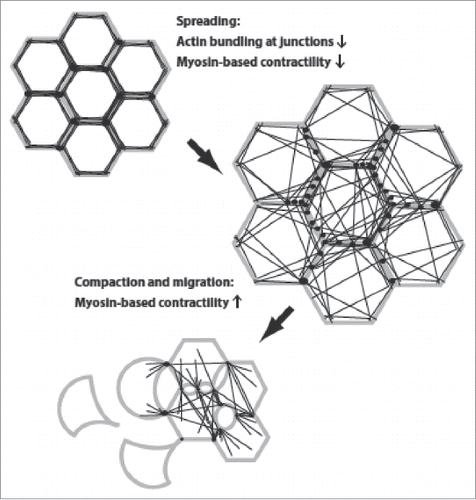
Figure 2. Regulation of actin dynamics by CaMKII. In its inactive state, CaMKII directly mediates actin bundling. Activation of CamKII, by calmodulin binding and subsequent phosphorylation reduces actin bundling by preventing CamKII-actin associations and by CaMKII-mediated phosphorylation of spinophilin and/or filamin. In addition, active CamKII impacts cellular contractility, either negatively (by phosphorylation and inhibition of myosin light chain kinase) or positively (by activating RhoA). CaMKII activation may thus act as a switch that alters global actin dynamics.
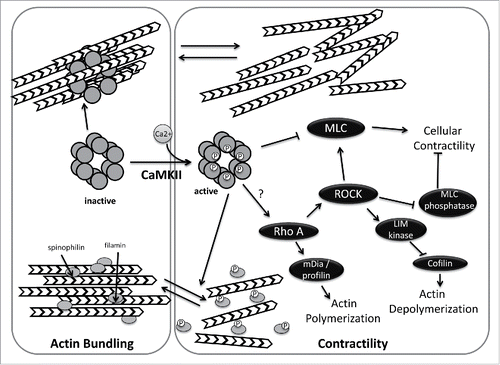
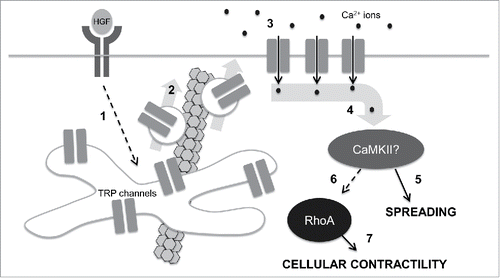
Figure 3. A speculative model for control of cell mechanics during epithelial scattering. In our model, 1.) HGF binding and activation of c-met receptor tyrosine kinases stimulates activates vesicle trafficking machinery in endosomal recycling compartments through an unidentified mechanism; 2.) Internal TRP channels are mobilized into vesicles that are destined for the plasma membrane in a microtubule-dependent vesicle trafficking step. 3.) TRP channels at the plasma membrane mediate a calcium influx that raises intracellular calcium concentrations. 4.) Intracellular calcium binds camodulin and in turn activates CaMKII. 5.) Early in epithelial scattering, CaMKII drives spreading, a result of reduced actin bundling at cell-cell junctions and myosin-based contractility. 6.) Late in epithelial scattering and through an as yet unidentified mechanism, CaMKII can stimulate an increase in RhoA activation. Seven. RhoA, through ROCK, increases myosin light chain phosphorylation, driving global cellular contractility and cell-cell detachment.