Figures & data
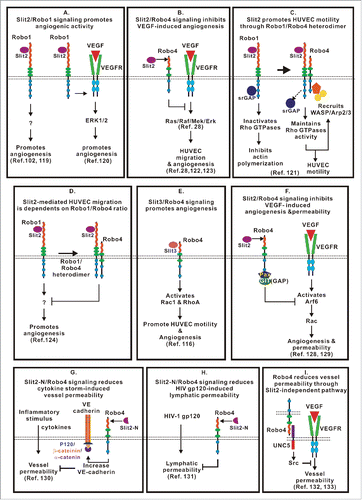
Figure 1. The domain structure of Slit and Robo proteins. (A) A schematic diagram of the domain organization of Slit1, Slit2 and Slit3 in humans. The domain structure of Slits includes 4 leucine-rich repeats (D1-D4), followed by 6 epidermal growth factor-like (EGF) domains, a laminin G domain, 3 additional EGF domains and a C-terminal cysteine knot. (B) A schematic diagram of the domain organization of Robo1, Robo2, Robo3 and Robo4 in vertebrates. The ectodomain is conserved among Robo1–Robo3, with 5 immunoglobulin (Ig) and 3 fibronectin type 3 (FNIII) domains. Robo4 contains only 2 Ig domains and 2 FNIII repeats. The intracellular domain of Robos contains several conserved motifs, including CC0-CC3 for Robo1–2, CC0 and CC2–3 for Robo3, and CC0 and CC2 for Robo4.
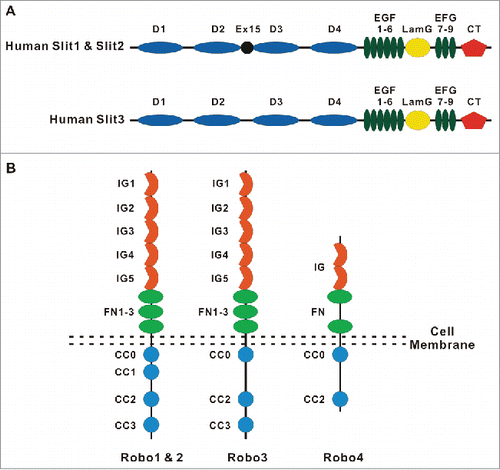
Figure 2. Molecular organization of intercellular junctions and cell-matrix adhesions. The Figure shows key adhesion structures formed by epithelial and endothelial cells. These structures include 2 junctional complexes, tight junctions and adherens junctions and as well as focal adhesions that mediate cell interactions with ECM. Tight junctions, adherens junctions and focal adhesions are linked to a prominent cortical actomyosin cytoskeleton that regulates the organization and dynamics of intercellular junctions and ECM adhesions.
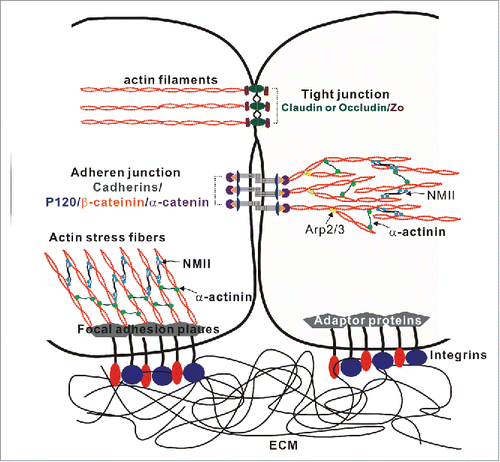
Figure 3. Roles of the Robo-Slit signaling and the development and functions of the glomerular filtration barrier. (A) Downregulation of Robo2 permits the interactions between the Wolffian duct (WD) and the nephrogenic cord (NC), resulting in the increased cell proliferation and formation of the ectopic ureteric bud (UB). This figure is adopted from CitationRef. 65. (B) Podocytes cover the outer surface of glomerular capillaries to form the glomerular filtration barrier that contains 3 structural elements: (1) a layer of fenestrated endothelial cells; (2) the glomerular basement membrane (GBM); (3) specialized epithelial cells with extended foot processes covering the outer face of the GBM. (C) The interplay between the Robo-Slit signaling and integrins mediates the establishing of podocyte-GBM adhesions. The laminin-binding integrins α3/β1 are linked to actin-based stress fibers through adaptor proteins. RhoA induces stress fiber formation through Rho-associated protein kinase (ROCK)-dependent phosphorylation of non-muscle myosin IIA (NMIIA). Slit2/Robo2 signaling inhibits NMIIA activity by sequestering myosin II regulatory light chain (MRLC) through srGAP1 (GAP) to the intracellular domain of Robo2. The “slit diaphragm” is the junction between adjacent podocyte foot processes. Nephrin is the major adhesive molecule within slit diaphragm that triggers actin polymerization through adaptor protein Nck. Slit2/Robo2 signaling inhibits nephrin-induced actin polymerization by forming the Robo2/Nck/nephrin complex.
Figure 4. Multiple roles of the Slit/Robo signaling in the regulation of intercellular junctions, ECM adhesion and cell motility. This figure summarizes current knowledge regarding signaling mechanisms initiated by Slit/Robo complexes and regulating integrity and remodeling of intercellular junctions and ECM adhesions. (A) Slit/Robo signaling inhibits actin polymerization through srGAP1 to inactivate Rho GTPases. (B) Slit1/Robo2 signaling inhibits N-cadherin-mediated cell adhesion by forming Robo2/N-cadherin complex through the Abl kinase and its adaptor proteins. The Abl kinase subsequently phosphorylates β-catenin resulting in its dissociation from the cadherin complex and nuclear translocation. (C) Slit1/Robo2 signaling induces E-cadherin degradation by recruiting the ubiquitin ligase Hakai to E-cadherin. (D) Slit1/Robo2 signaling enhances the formation of the E-cadherin/β-catenin complex through AKT/GSK3β/Snail pathway. (E) Slit2/Robo3 signaling promotes the interaction between Robo3 and P-cadherin, which, in turn, enhances the formation of E-cadherin homodimer and inhibits cell migration.
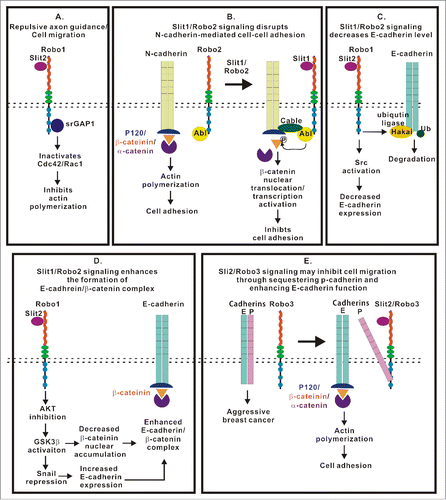
Figure 5. Complex and context dependent functions of the Slit/Robo signaling in angiogenesis. (A) Slit2/Robo1 signaling promotes angiogenesis by activating Robo1-EGFR2-ERK1/2 signaling or by yet to be determined intracellular events. (B) Slit2/Robo4 signaling blocks VEGF-induced angiogenesis by inhibiting the Ras/Raf/Mek/Erk pathway. (C) Slit2 binds to the Robo1/Robo4 heterodimer to promote HUVEC motility by recruiting WASP and Arp2/3 for actin polymerization. (D) Assembly of the Robo1/Robo4 heterodimer reduces Slit2/Robo1 signaling-mediated pro-angiogenesis activity. (E) Slit3/Robo4 signaling activates Rho GTPases and promotes HUVEC motility and angiogenesis. (F) Slit2/Robo4 signaling activates GIT/GAP and inhibits VEGF-induced permeability of blood vessels and angiogenesis by inactivating Arf6 GTPase. (G) Slit2N/Robo4 signaling inhibits endothelial permeability caused by cytokine production in sepsis and influenza by increasing VE-cadherin recruitment to the plasma membrane. (H) The Slit2N/Robo4 signaling attenuates increased permeability of lung lymphatic vessels caused by HIV-1-derived gp120 glycoprotein. (I) Robo4 stabilizes blood vessel through Slit2-independent pathway. Intercellular interaction of Robo4 and UNC5 inhibits VEGF-induced vessel permeability.