Figures & data
Table 1. Targeting various diseases by curcumin supplementation. A large number of clinical trials have been conducted to evaluate the beneficial effects of Curcumin supplementation and have been summarized by Gupta et al.Citation86 Listed below are some of the recent trials for modulation of targeted diseases.
Figure 1. The four layers of the Intestinal barrier. (1) Intestinal alkaline phosphatase (IAP) secreted by the intestinal epithelial cells constitutes the first or the luminal layer and it detoxifies LPS; (2) Loosely attached outer mucin layer and the firmly attached inner mucin layer form the second layer of the barrier and are involved in restricting entry of pathogenic bacteria thereby preventing direct contact of bacteria with the intestinal epithelial cells; (3) Intestinal epithelial cells with tight junctions regulate the transcellular or paracellular transport from the lumen to the systemic circulation (4) Paneth cells located in the crypts produce antibacterial proteins the block any bacteria that have penetrated the overlying layers of the barrier.
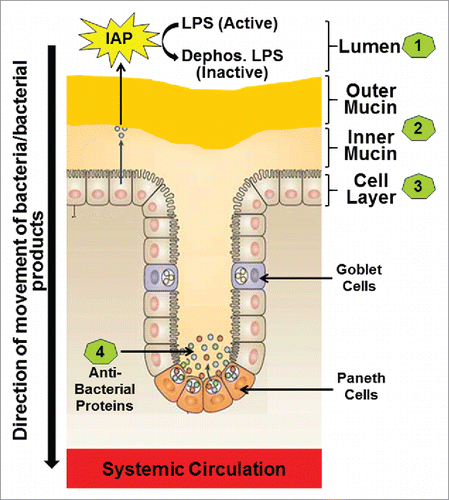
Figure 2. Organization of the tight junctions. Membrane spanning proteins Occludin, Claudins, Junctional adhesion molecules (JAMs) and CAR form the fibrils connecting two adjacent cells. The intracellular domains of these proteins are associated with ZO-1, ZO-2 and ZO-3 and also connected to the actin microfilaments. This organization of these tight junction proteins is crucial to maintaining the paracellular transport and is disrupted by changes in expression or phosphorylation of these proteins as well as disorganization of actin microfilaments.
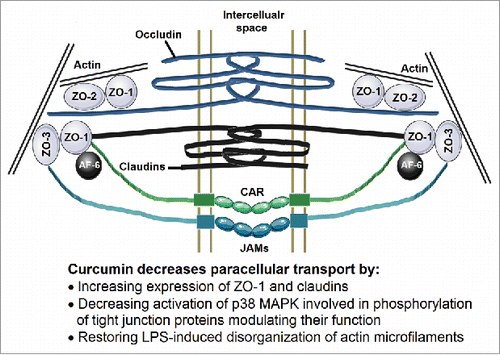
Figure 3. High fat high cholesterol containing Western diet (WD)-mediated disruption of intestinal barrier function and its systemic consequences. WD decreases the activity of intestinal alkaline phosphatase (IAP), disrupts the mucin layers and increase inflammation of intestinal epithelial cell layer by enhancing infiltration of macrophages. Collectively, these effects lead to increased paracellular permeability and lead to an increase in circulating LPS levels. Systemic and tissue macrophages are activated in response to this metabolic endotoxemia and lead to a chronic inflammatory state that underlie the development of multiple diseases including chronic kidney disease (CKD). Infiltration of activated macrophages in adipose tissue or artery wall lead to the development of Type 2 Diabetes (T2DM) or atherosclerosis, respectively.
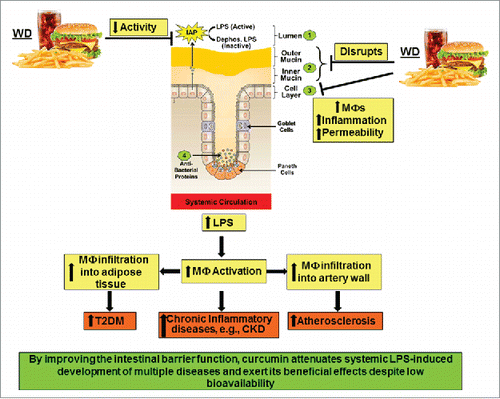
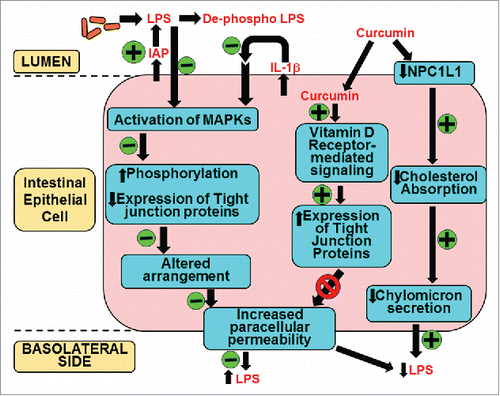
Figure 4. Mechanism(s) of action of curcumin in systemic LPS levels. Oral supplementation with curcumin increases the activity of intestinal alkaline phosphatase (IAP) enhancing luminal deactivation of LPS. De-phospho LPS acts as a TLR4 antagonist further reducing LPS-TLR4 interaction and downstream signaling resulting in reduced secretion of pro-inflammatory cytokine IL-1β. As a result LPS or IL-1β mediated activation of MAPK and subsequent phosphorylation and expression of tight junction proteins is attenuated. These modulatory effects of curcumin prevent the disruption of tight junction organization and decrease LPS-mediated increase in paracellular permeability. Additionally, curcumin is taken up by the intestinal epithelial cells and via binding to the vitamin D receptor initiates intracellular signaling events leading to an increase in the expression of tight junction proteins that reduce paracellular permeability. Luminal LPS is also translocated bound to chylomicrons and curcumin decreases cholesterol absorption by reducing the expression of apical cholesterol transporter NPC1L1 resulting in attenuated chylomicron secretion. This pathway may represent yet another mechanism by which curcumin can decrease systemic LPS levels. All events negatively or positively regulated by curcumin are indicated by “-“ or “+” sign in the green circles, respectively.