Figures & data
Table 1. Overview of targets expressed by both the TR146 cell line and the biopsy samples upon PCR or real-time PCR showing nine different categories of markers. Abbreviations are explained below the table (all biopsy samples additionally expressed tight junction protein CLDN5, 5 out of 8 samples expressed MUC19).
Figure 1. (A) TEER value progressions of submerged and airlift cultivation in final EpiLife media (E3) shown as Ω x cm2 (mean ± SEM, n = 3–12 inserts from four independent experiments). Statistical analysis was performed with two-way ANOVA following post hoc Tukey's test, with α = 0.05, ***p < 0.001. (B) Permeability coefficient values [µm/min] of carboxyfluorescein on the last day of experiments after cultivation in final EpiLife media (E3) (mean ± SD, n = 8–9 inserts from three independent experiments). Statistical analysis was performed with Student’s t-test (airlift vs submerged), α = 0.05, ***p < 0.001. (C) TEER value progressions of submerged and airlift cultivation in DMEM media shown as Ω x cm2 over time (mean ± SEM, n = 3–18 inserts from six independent experiments). Statistical analysis was performed with two-way ANOVA following Holm–Sidak as post hoc test, with α = 0.05, *p <0.05, **p < 0.01 ***p < 0.001. (D) Permeability coefficient values [µm/min] of carboxyfluorescein on the last day of experiments after cultivation in DMEM media under submerged and airlift condition (mean ± SD, n = 15 inserts from five independent experiments).
![Figure 1. (A) TEER value progressions of submerged and airlift cultivation in final EpiLife media (E3) shown as Ω x cm2 (mean ± SEM, n = 3–12 inserts from four independent experiments). Statistical analysis was performed with two-way ANOVA following post hoc Tukey's test, with α = 0.05, ***p < 0.001. (B) Permeability coefficient values [µm/min] of carboxyfluorescein on the last day of experiments after cultivation in final EpiLife media (E3) (mean ± SD, n = 8–9 inserts from three independent experiments). Statistical analysis was performed with Student’s t-test (airlift vs submerged), α = 0.05, ***p < 0.001. (C) TEER value progressions of submerged and airlift cultivation in DMEM media shown as Ω x cm2 over time (mean ± SEM, n = 3–18 inserts from six independent experiments). Statistical analysis was performed with two-way ANOVA following Holm–Sidak as post hoc test, with α = 0.05, *p <0.05, **p < 0.01 ***p < 0.001. (D) Permeability coefficient values [µm/min] of carboxyfluorescein on the last day of experiments after cultivation in DMEM media under submerged and airlift condition (mean ± SD, n = 15 inserts from five independent experiments).](/cms/asset/5d1c5fd4-31f8-42c7-92f3-8acd542879e0/ktib_a_1748459_f0001_b.gif)
Figure 2. Cultivation of TR146 cells on inserts in DMEM with supplements monitored with TEER and permeability of carboxyfluorescein (A) Progression of TEER values versus time as x-fold. Ω x cm2 values of submerged cultivation in DMEM media (●) was used as reference for airlift cultivation in DMEM media (○), supplemented with 10 (▼), 100 (∆) or 1000 nM (□) HC. Statistical analysis was performed as two-way ANOVA with Holm–Sidak test (mean ± SD, n = 9 inserts from three independent experiments, α = 0.05, *p < 0.05, **p < 0.01, ***p < 0.001). (B) Corresponding permeability coefficients of carboxyfluorescein on the last day of experiments (mean ± SD, n = 9 inserts from three independent experiments, one-way ANOVA, Tukey test) to 2A. (C) Comparison of submerged cultivation in DMEM (●) to airlift in DMEM (○), supplemented with 1% HKGS (▼) or HKGS/KGF/A2P (∆) (mean ± SD, n = 9–12 inserts from three independent experiments, two-way ANOVA with Holm–Sidak test). (D) Permeability values of carboxyfluorescein on the last day of experiments corresponding to 2C (mean ± SD, n = 8 inserts from two independent experiments, one-way ANOVA, Dunn´s test).
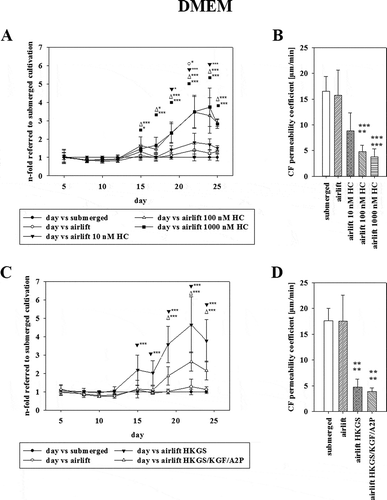
Figure 3. (A) Effect of serum deprivation on TEER using corresponding Ω x cm2 values of airlift cultivation in DMEM supplemented with HKGS (●) containing 10% serum as reference. DMEM without serum (○), supplemented with HKGS (▼) or HKGS/KGF/A2P (∆) was tested (mean ± SD, n = 3–6 inserts from three independent experiments, two-way ANOVA with Holm–Sidak test). (B) Permeability values of carboxyfluorescein on the last day of experiments corresponding to 3A (mean ± SD, n = 3–6 inserts from three independent experiments, one-way ANOVA).
Figure 4. Expression values of (A) tight junction proteins, (B) transporter proteins, and (C,) receptors of the High-Throughput qPCR Chip shown as ∆Ct values, referred to PPIA (peptidylprolyl isomerase A) as endogenous control. TR146 samples [Samples 1–39, 1–3: cultivated in EpiLife media (E3), submerged; 4–6: EpiLife (E3), airlift; 7–11: DMEM media, submerged; 12–16: DMEM media, airlift; 17–19: DMEM media supplemented with 1% HKGS, airlift; 20–22: DMEM media supplemented with HKGS/KGF/A2P, airlift; 23–25: DMEM media supplemented with 10 nM HC, airlift; 26–28: DMEM media supplemented with 100 nM HC, airlift: 29–31: DMEM media supplemented with 1000 nM HC, airlift: 32–33: DMEM media w/o serum, airlift; 34–36: DMEM media w/o serum supplemented with 1% HKGS, airlift: 36–39: DMEM media w/o serum supplemented with HKGS/KGF/A2P, airlift] plotted against 8 biopsy samples (B1-8) of the oral mucosa.
![Figure 4. Expression values of (A) tight junction proteins, (B) transporter proteins, and (C,) receptors of the High-Throughput qPCR Chip shown as ∆Ct values, referred to PPIA (peptidylprolyl isomerase A) as endogenous control. TR146 samples [Samples 1–39, 1–3: cultivated in EpiLife media (E3), submerged; 4–6: EpiLife (E3), airlift; 7–11: DMEM media, submerged; 12–16: DMEM media, airlift; 17–19: DMEM media supplemented with 1% HKGS, airlift; 20–22: DMEM media supplemented with HKGS/KGF/A2P, airlift; 23–25: DMEM media supplemented with 10 nM HC, airlift; 26–28: DMEM media supplemented with 100 nM HC, airlift: 29–31: DMEM media supplemented with 1000 nM HC, airlift: 32–33: DMEM media w/o serum, airlift; 34–36: DMEM media w/o serum supplemented with 1% HKGS, airlift: 36–39: DMEM media w/o serum supplemented with HKGS/KGF/A2P, airlift] plotted against 8 biopsy samples (B1-8) of the oral mucosa.](/cms/asset/8278746b-c44f-4704-8cc2-99407a08f7a4/ktib_a_1748459_f0004_oc.jpg)
Figure 5. Expression values of (A) cytokeratins, cornification, and EMT (epithelial–mesenchymal transition) markers (B) mucins and (C) aquaporins of the High-Throughput qPCR Chip shown as ∆Ct values, referred to PPIA (peptidylprolyl isomerase A) as endogenous control. TR146 samples [Samples 1–39, 1–3: cultivated in EpiLife media (E3), submerged; 4–6: EpiLife (E3), airlift; 7–11: DMEM media, submerged; 12–16: DMEM media, airlift; 17–19: DMEM media supplemented with 1% HKGS, airlift; 20–22: DMEM media supplemented with HKGS/KGF/A2P, airlift; 23–25: DMEM media supplemented with 10 nM HC, airlift; 26–28: DMEM media supplemented with 100 nM HC, airlift: 29–31: DMEM media supplemented with 1000 nM HC, airlift: 32–33: DMEM media w/o serum, airlift; 34–36: DMEM media w/o serum supplemented with 1% HKGS, airlift: 36–39: DMEM media w/o serum supplemented with HKGS/KGF/A2P, airlift] plotted against 8 biopsy samples (B1-8) of the oral mucosa.
![Figure 5. Expression values of (A) cytokeratins, cornification, and EMT (epithelial–mesenchymal transition) markers (B) mucins and (C) aquaporins of the High-Throughput qPCR Chip shown as ∆Ct values, referred to PPIA (peptidylprolyl isomerase A) as endogenous control. TR146 samples [Samples 1–39, 1–3: cultivated in EpiLife media (E3), submerged; 4–6: EpiLife (E3), airlift; 7–11: DMEM media, submerged; 12–16: DMEM media, airlift; 17–19: DMEM media supplemented with 1% HKGS, airlift; 20–22: DMEM media supplemented with HKGS/KGF/A2P, airlift; 23–25: DMEM media supplemented with 10 nM HC, airlift; 26–28: DMEM media supplemented with 100 nM HC, airlift: 29–31: DMEM media supplemented with 1000 nM HC, airlift: 32–33: DMEM media w/o serum, airlift; 34–36: DMEM media w/o serum supplemented with 1% HKGS, airlift: 36–39: DMEM media w/o serum supplemented with HKGS/KGF/A2P, airlift] plotted against 8 biopsy samples (B1-8) of the oral mucosa.](/cms/asset/dad5e636-a5ab-4e8f-b7c6-d61131bc05c1/ktib_a_1748459_f0005_oc.jpg)
Table 2. Comparison of highly expressed targets in human oral biopsy samples (n = 8) versus TR146 cells cultivated under optimized conditions (DMEM media, supplemented with HKGS, airlift, n = 3, final TEER of 115.39 ± 38.48 Ω x cm2). Ct values were obtained with the 96.96 High-Throughput qPCR-Chip, referred to PPIA (peptidylprolyl isomerase A) as housekeeping gene, normalized to the expression values of biopsy samples and listed as x-fold values (mean ± SEM). Statistical analysis was performed using the Student´s t-test with α = 0.05, *p <0.05, **p < 0.01, ***p < 0.001.
Figure 6. Representative western blots of TR146 cells cultivated in optimized media under airlift conditions in comparison to a control sample (immortalized mouse capillary endothelial cell line cerebEND cultivated as previously publishedCitation18) showing the expression of ABC transporters (A) P -gp (ABCB1, 170 kDa), (B) MRP4 (ABCC4, 140–200 kDa), (C) BCRP (ABCG2, 72-75 kDa); corresponding ß-actin (42 kDa) was used as loading control.
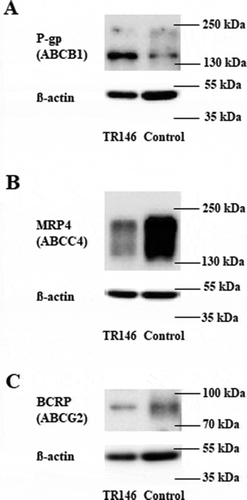
Table 3. Results of ABC transporter functionality tests in TR146 cells. For each assay at least 6 values were obtained for each condition (control, control with fluorescent substrate, samples of fluorescent substrate with or without inhibitor). The calculated ratios of fluorescent signal to protein content were normalized to samples only exposed to the fluorescent substrate (set as 100%). An active inhibition is noticeable by a significantly increased uptake of the substrate (>100%). Values are shown for both DMEM and EpiLife media as mean ± SD. Data were analyzed with Student’s t-test or with one-way ANOVA with post hoc comparison using Dunn´s method and Holm–Sidak test for multigroup comparison, **p < 0.01, ***p < 0.001, SC = statistical significance, n/a = not analyzed, n.s. = not significant, n = number of independently conducted experiments.
